An Integrative Review of Sex Differences in Quality of Life and Symptoms Among Survivors of Hematologic Malignancies
Objectives: To conduct an integrative review of studies to identify disparities in quality of life (QOL), symptoms, and symptom burden between men and women diagnosed with hematologic malignancies.
Sample & Setting: 11 studies comprising 13,546 participants aged 18 years or older were included in the analysis. Studies were original peer-reviewed research published in English between January 2005 and December 2020.
Methods & Variables: A literature search was performed using keywords associated with health-related QOL, hematologic malignancy, and sex/gender differences. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed to identify relevant studies. Data were extracted for sex differences in QOL, symptoms, and symptom burden. All studies were appraised for quality and level of evidence.
Results: Women have worse physical health and function, more pain, and higher symptom burden compared with men.
Implications for Nursing: Healthcare providers need to understand the impact of sex-based differences on QOL, symptoms, and symptom burden to provide optimal, personalized care.
Jump to a section
An estimated 1.96 million Americans will be newly diagnosed with cancer in 2023 (Siegel et al., 2023). According to the American Cancer Society, 184,720 individuals in the United States will be diagnosed with hematologic malignancies such as leukemia, lymphoma, or myeloma in 2023 (Siegel et al., 2023). A cancer diagnosis is frequently associated with multiple symptoms that vary depending on cancer type and stage of disease. In addition, radiation therapy, chemotherapy, and surgery trigger an array of side effects that may affect the quality of life (QOL) of patients with cancer. There are sex-based differences in the bioavailability, enzyme expression, and chemical mechanisms of drug therapy for oncologic diseases (Schmetzer & Flörcken, 2012). Several studies in hematology-oncology report varying differences in side effects and survival rates between sexes, validating the need for a better understanding of how QOL differs between men and women diagnosed with hematologic malignancies (Klimm et al., 2005; Molica, 2006; Pfreundschuh et al., 2014).
In a large study of 10,700 symptom assessments focused on performance status trajectories and symptom scores in patients with cancer during the final six months of life, four symptoms (drowsiness, tiredness, well-being, and lack of appetite) were reported as moderate to severe (Seow et al., 2011). Fifty percent of the participants in this study were female. Researchers used multivariate analysis to assess the likelihood that participants would report a moderate to severe symptom score. Female participants were significantly more likely to report moderate to severe symptoms of nausea, anxiety, drowsiness, poor well-being, lack of appetite, and tiredness (Seow et al., 2011). This study established the importance of and need for additional research to increase the understanding of sex-based disparities in QOL, symptoms, and symptom burden for individuals diagnosed with a hematologic malignancy.
The primary aim of this article is to provide a comprehensive review of studies published in peer-reviewed journals that investigate how QOL, symptoms, and symptom burden differ between women and men diagnosed with hematologic malignancies. The research questions posed are as follows:
- How do symptoms of men and women diagnosed with a hematologic malignancy differ?
- Does symptom burden differ between men and women diagnosed with a hematologic malignancy?
- Based on the symptoms that are experienced, does QOL differ between men and women diagnosed with a hematologic malignancy?
Methods
Electronic Database Searches
Electronic database searches were performed in November 2020 with the support of a medical librarian specializing in consumer health information. Citations from search results were downloaded into Covidence and subsequently imported into the reference manager software EndNote X9.
Studies published from January 2005 to December 2020 were included in the search using PubMed®, CINAHL®, and PsycINFO®. A span of 15 years was necessary to form a foundation for the research questions because of limited relevant publications. The authors used Medical Subject Headings (MeSH) terms and keywords related to sex-based QOL differences in patients with hematologic malignancies. Combination terms of QOL, health-related QOL (HRQOL), neoplasm, hematologic malignancy, and sex/gender differences were used. Additional publications were included after reviewing the reference lists of eligible articles. All abstracts and publications were reviewed by two investigators. When investigators disagreed on the inclusion of articles, they discussed the articles until they came to a resolution.
Studies in this integrative review met the following inclusion criteria: (a) being randomized controlled trials or qualitative studies focused on sex-based differences in QOL, symptoms, or symptom burden between men and women with a hematologic malignancy; (b) containing a study sample aged 18 years or older; (c) being original research published in English; (d) being published between January 2005 and December 2020; and (e) being published in peer-reviewed journals. Exclusion criteria for studies were as follows: (a) being abstracts or results of dissertations; (b) not reporting separate outcomes for QOL in men and women; (c) being published before January 1, 2005; or (d) focusing on a pediatric population. For this review, the terms “sex” and “gender” were used interchangeably, although the authors acknowledge sex as a biologic variable and gender as a set of socially allocated roles and expectations associated with being designated either male or female.
Search Outcomes
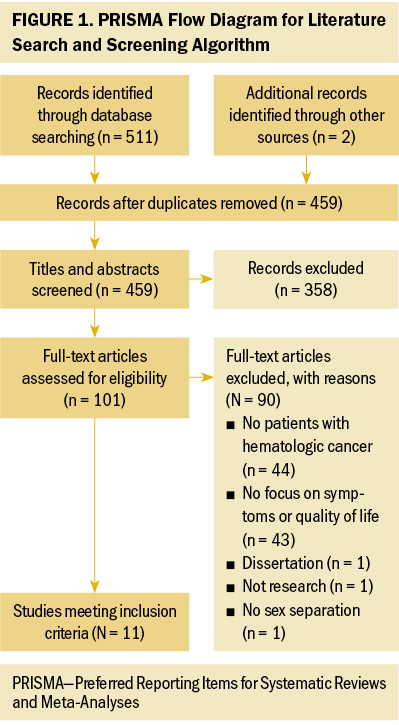
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram was used to screen and remove manuscripts (see Figure 1). Overall, 511 records were initially screened from database searches, and 2 were identified from reference lists of eligible studies. After removing 54 duplicates, 459 studies were screened against the titles and abstracts, with 358 studies excluded. The full texts of the remaining 101 studies were screened for eligibility. Ninety studies were excluded for the following reasons: (a) 44 did not include patients with hematologic cancers, (b) 43 lacked focus on symptoms or QOL, (c) 1 was part of a dissertation, (d) 1 was not a study, and (e) 1 did not separate data by sex. This resulted in 11 studies.
Data Extraction, Level of Evidence, and Quality Appraisal
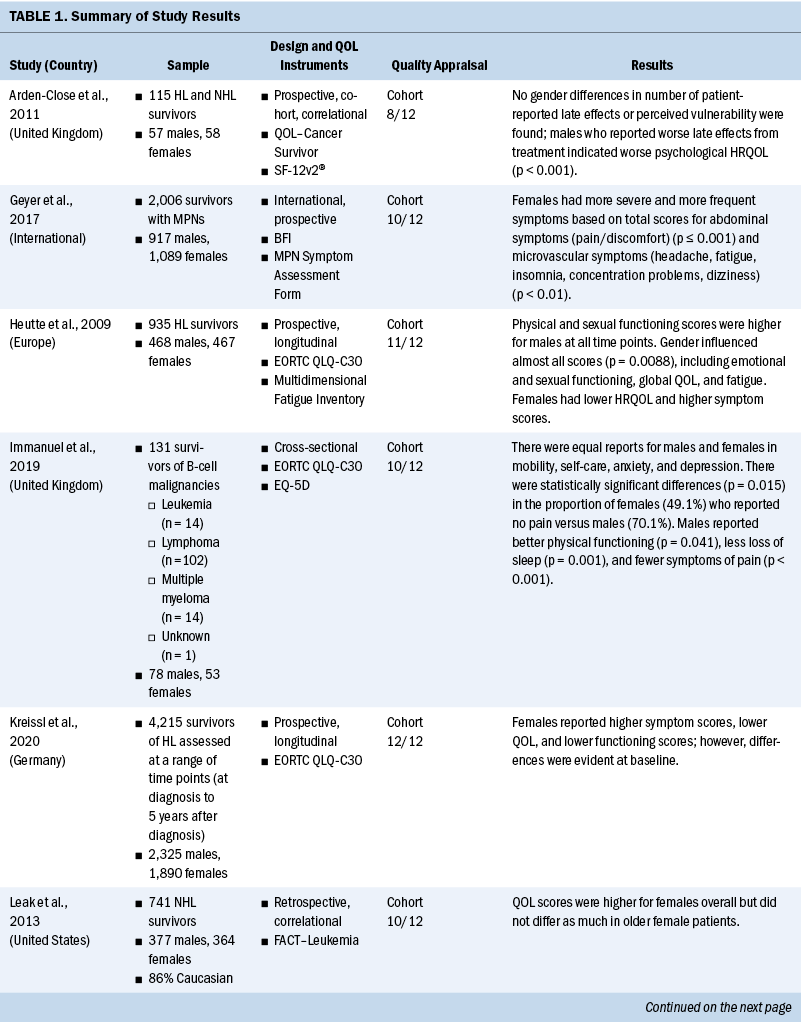
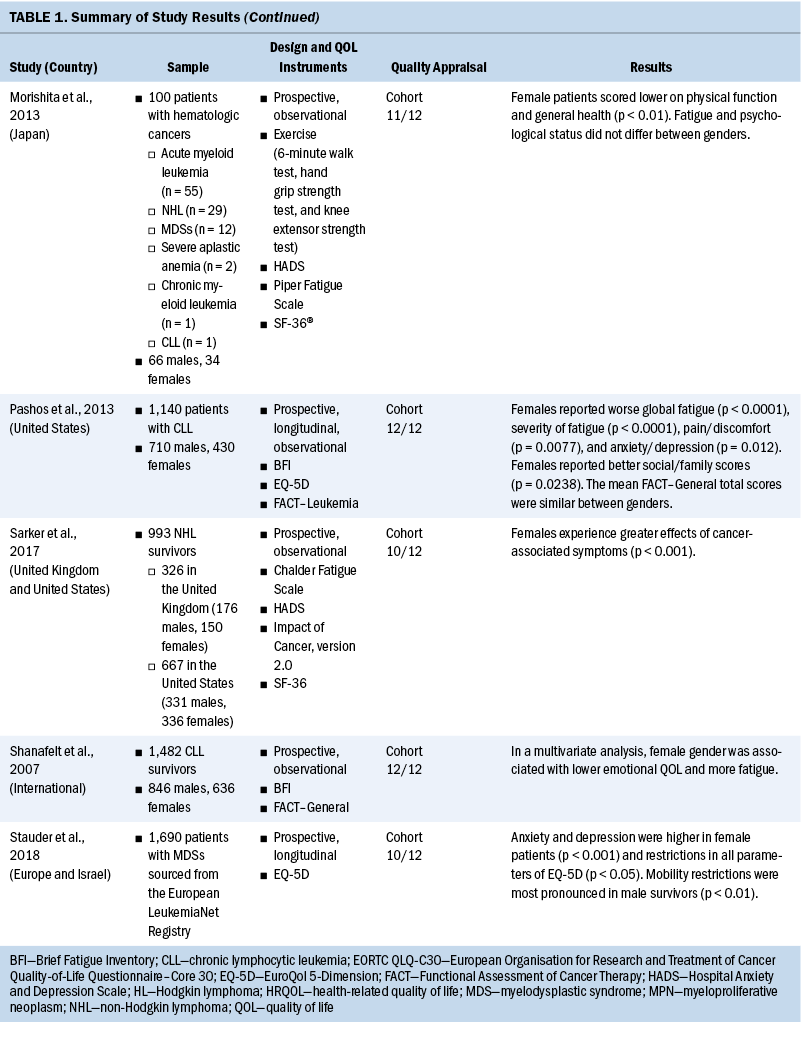
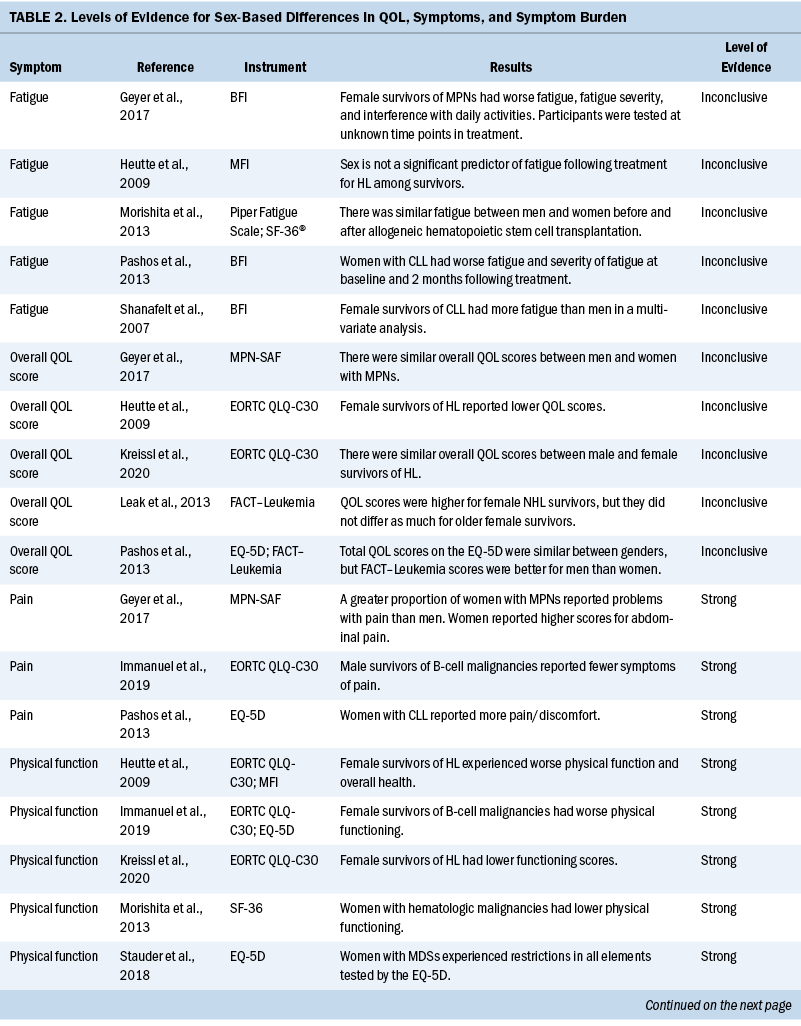
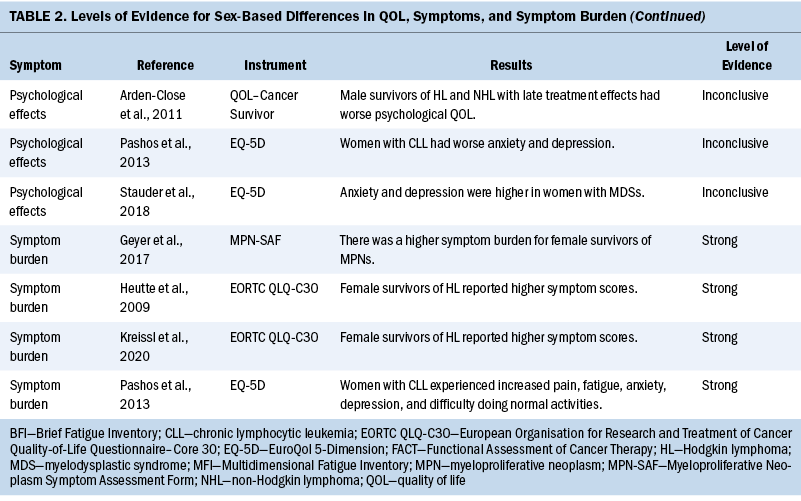
Data related to study authors and setting, sample, study design, measurement instruments, quality appraisal, and results were extracted (see Table 1). Results related to QOL, symptoms, and symptom burden were synthesized, and levels of evidence were assessed (see Table 2). Level of evidence was defined as strong if two or more high-quality studies provided consistent findings, moderate if only one high-quality study provided findings, inconclusive if there were inconsistent findings regardless of the study quality, and no evidence if no associations were found.
All studies were reviewed using the Critical Appraisal Skills Programme (2022) checklists according to study design to assess methodologic quality, presence of bias, degree of uncertainty in the results, and relevance of the findings. The first and second authors independently used these checklists to record whether each item had been reported adequately. Based on appraisals, studies were then reported using the Cohort Study Checklist, and the number of criteria that each study met was determined (Critical Skills Appraisal Programme, 2022). A consensus was reached by the authors on whether to include each study.
Data Analysis
Because of the heterogeneity of study design, re-search questions, and QOL instruments used in the studies in this review, a narrative synthesis approach was adopted. This approach involves grouping results to examine similarities and differences and synthesizing them into a coherent narrative. This allowed related information to be collected and organized in relation to sex-related differences in QOL, symptoms, and symptom burden. Meaningful conclusions were drawn using ideas webbing (Clinkenbeard, 1991) and concept mapping (Mulrow et al., 1997) techniques.
Results
Study Characteristics
Among the 11 studies, 9 were prospective (Arden-Close et al., 2011; Geyer et al., 2017; Heutte et al., 2009; Kreissl et al., 2020; Morishita et al., 2013; Pashos et al., 2013; Sarker et al., 2017; Shanafelt et al., 2007; Stauder et al., 2018), and 4 of these 9 were longitudinal (Heutte et al., 2009; Kreissl et al., 2020; Pashos et al., 2013; Stauder et al., 2018). Only one cross-sectional study (Immanuel et al., 2019) and one retrospective study (Leak et al., 2013) were included.
Measurement Instruments
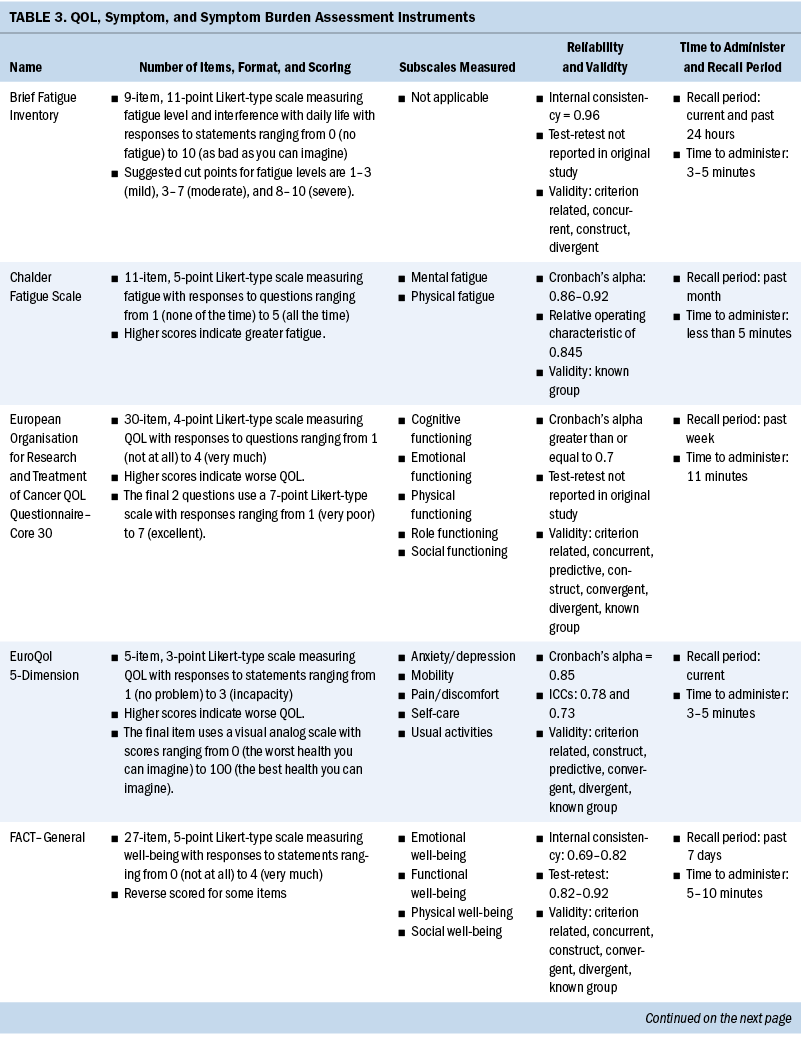
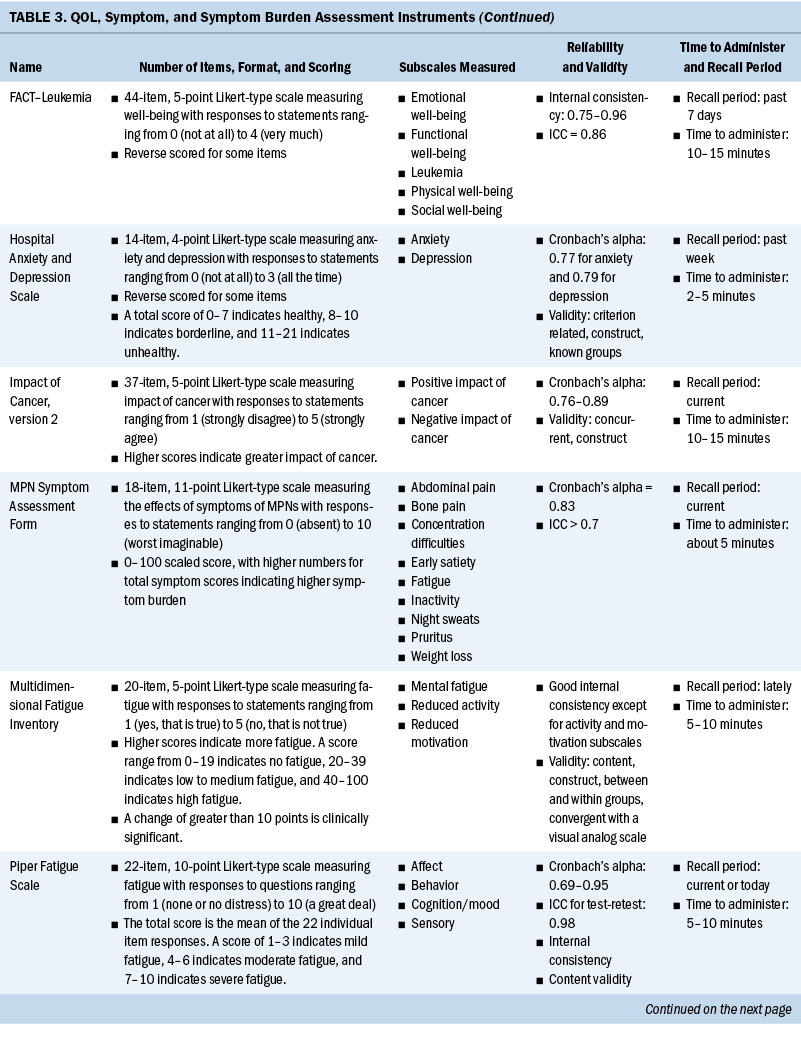
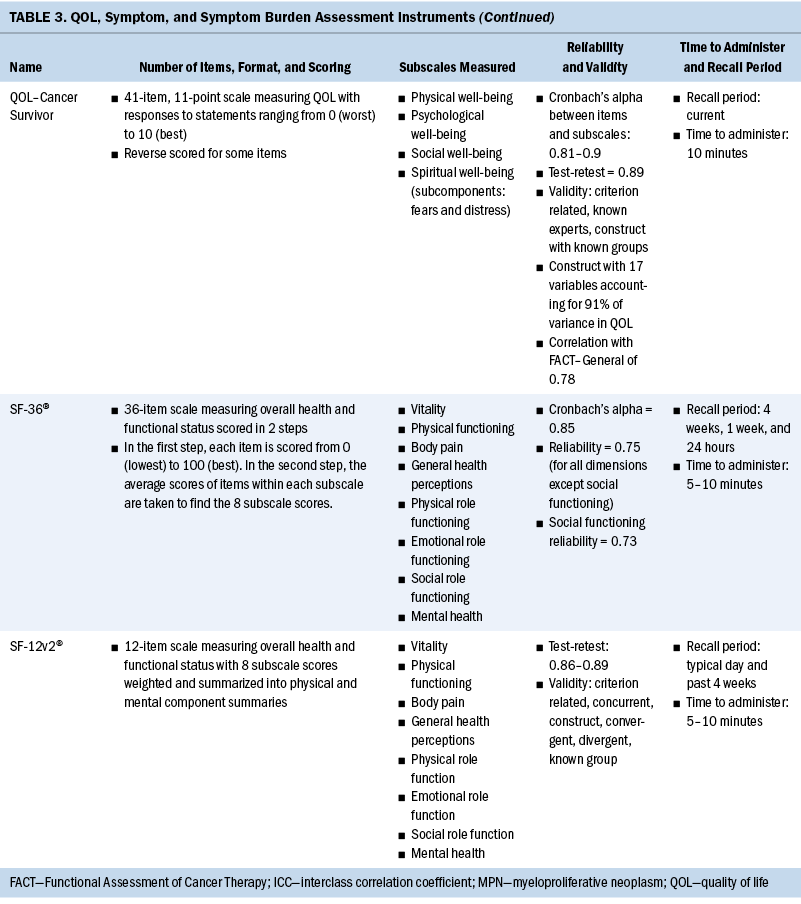
Measurement of QOL and symptoms varied within each study. Three studies used the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 (EORTC QLQ-C30) instrument (Heutte et al., 2009; Immanuel et al., 2019; Kreissl et al., 2020). The EuroQol 5-Dimension (EQ-5D), a generic instrument to measure QOL, was used in three of the studies (Immanuel et al., 2019; Pashos et al., 2013; Stauder et al., 2018). The Functional Assessment of Cancer Therapy (FACT)–General (FACT-G) and the leukemia-specific FACT–Leukemia (FACT-Leu) were used in three studies to measure QOL (Leak et al., 2013; Pashos et al., 2013; Shanafelt et al., 2007). Geyer et al. (2017) developed their own instrument for a study evaluating common symptoms associated with myeloproliferative neoplasms (MPNs), the MPN Symptom Assessment Form (MPN-SAF). The SF-36® and the 12-item version of this survey, the 12-item SF-12v2®, were used in three studies (Arden-Close et al., 2011; Morishita et al., 2013; Sarker et al., 2017) to measure functional health and well-being. One study used the QOL–Cancer Survivor instrument to measure HRQOL (Arden-Close et al., 2011).
Several instruments were used to measure fatigue. The Brief Fatigue Inventory (BFI) was the most frequently used instrument (Geyer et al., 2017; Pashos et al., 2013; Shanafelt et al., 2007). Other fatigue scales used were the Piper Fatigue Scale (Morishita et al., 2013), the Multidimensional Fatigue Inventory (MFI) (Heutte et al., 2009), and the Chalder Fatigue Scale (Sarker et al., 2017). See Table 3 for a full comparison of instruments used to measure QOL, symptoms, and symptom burden.
Participant Characteristics
From the 11 studies, there were 13,546 participants, consisting of 6,158 women and 7,388 men. Several different hematologic malignancies were investigated, with Hodgkin lymphoma and non-Hodgkin lymphoma reported in nine studies (Arden-Close et al., 2011; Heutte et al., 2009; Immanuel et al., 2019; Kreissl et al., 2020; Leak et al., 2013; Morishita et al., 2013; Pashos et al., 2013; Sarker et al., 2017; Shanafelt et al., 2007). One study concentrated on MPNs (Geyer et al., 2017), and one study included only patients diagnosed with myelodysplastic syndromes (MDSs) (Stauder et al., 2018). Acute leukemias were not well represented, with data from only 69 patients included. This review identifies vital knowledge gaps around sex differences in QOL, symptoms, and symptom burden experienced by patients with acute or chronic leukemia.
QOL, Symptom, and Symptom Burden Differences
QOL: Differences in QOL between men and women among survivors of hematologic malignancies were inconclusive (Geyer et al., 2017; Heutte et al., 2009; Kreissl et al., 2020; Leak et al., 2013; Pashos et al., 2013). Three studies (Geyer et al., 2017; Kreissl et al., 2020; Pashos et al., 2013) showed similar overall QOL scores between sexes. In contrast, Heutte et al. (2009) reported that among survivors of Hodgkin lymphoma, women had lower QOL scores than men on the EORTC QLQ-C30 following treatment. Leak et al. (2013) reported that women scored higher than men on the FACT-Leu for overall QOL. These measurements were taken after the completion of treatment in survivors of non-Hodgkin lymphoma.
Fatigue: Staging and treatment are different for patients with diagnoses of hematologic malignancies than for patients with solid tumors. However, both groups experience fatigue as a common troubling symptom that interferes with QOL (Hofer et al., 2018). Five studies identified sex-based differences in fatigue, with conflicting results (Geyer et al., 2017; Heutte et al., 2009; Morishita et al., 2013; Pashos et al., 2013; Shanafelt et al., 2007). Pashos et al. (2013) noted that fatigue was worse, more severe, and more hindering to activities among women. This study was focused on patients with chronic lymphocytic leukemia (CLL) initiating treatment. It comprised 1,140 patients, with females representing 38% (n = 430) of the sample. Fatigue was measured using the BFI. Shanafelt et al. (2007) found similar results in patients with CLL using multivariate analysis that controlled for different cancer stages, with women reporting more fatigue than men. Overall, 40% had been treated with chemotherapy or monoclonal antibody–based therapy, and 9% were receiving active treatment (Shanafelt et al., 2007). Research on patients with MPNs found fatigue as measured by the BFI to have greater severity, higher frequency, and more interference with activities among women (Geyer et al., 2017). A study by Morishita et al. (2013) found similar fatigue scores on the Piper Fatigue Scale between men and women before and after allogeneic hematopoietic stem cell transplantation. In a separate study, Heutte et al. (2009) found fatigue among survivors of Hodgkin lymphoma as measured by the MFI not to be predicted by sex. In terms of fatigue, the results are inconclusive for sex differences, with three studies suggesting that fatigue is increased in women compared with men. Two studies of patients with CLL found that women reported more fatigue. The other study results may have been confounded by disease and setting, showing similar fatigue scores for men and women.
Physical functioning: Patients with hematologic malignancies often experience symptoms that affect physical functioning. In this review, physical functioning was measured using the EORTC QLQ-C30, EQ-5D, MFI, and SF-36 (Heutte et al., 2009; Immanuel et al., 2019; Kreissl et al., 2020; Morishita et al., 2013; Stauder et al., 2018). Women reported reduced physical functioning than men in five studies (Heutte et al., 2009; Immanuel et al., 2019; Kreissl et al., 2020; Morishita et al., 2013; Stauder et al., 2018). The results from these studies were consistent, providing strong evidence that women have reduced physical functioning associated with a hematologic malignancy diagnosis compared with men. Immanuel et al. (2019) found that physical functioning among survivors of hematologic malignancies was reduced in women compared with men. Morishita et al. (2013) found that in the early phase following hematopoietic stem cell transplantation, scores for HRQOL and physical functioning as measured by knee extensor strength, hand grip strength, and the six-minute walk test, were significantly lower for women than for men (p < 0 .01). Heutte et al. (2009) conducted research focused on 935 survivors of Hodgkin lymphoma with more than 2,516 assessments performed during the study, which found that female survivors had lower physical functioning scores than male survivors at all time points. Kreissl et al. (2020) also examined the physical symptoms throughout the cancer trajectory in survivors of Hodgkin lymphoma from the time of diagnosis to five years into survivorship. They found that women reported lower functional scores than men on the EORTC QLQ-C30. Stauder et al. (2018) reported that being female with low- to intermediate-1–risk MDS was significantly associated with decreased scores on the EQ-5D index (p < 0.001). In all studies for this review, women reported reduced physical functioning in comparison to men.
Psychological effects: The review of differences between men and women in psychological effects among patients with hematologic malignancies was inconclusive, with three studies (Arden-Close et al., 2011; Pashos et al., 2013; Stauder et al., 2018) reporting sex-based differences. In a prospective, observational study, Pashos et al. (2013) compared 710 males with 430 females diagnosed with CLL. Women (35.3%) reported greater anxiety/depression than men (27.8%) on the EQ-5D (p = 0.012). The patients were measured at baseline and within two months of starting treatment for CLL. On average, women (mean = 71 years, SD = 11 years) were older than men (mean = 68 years, SD = 11 years), and it is unclear whether this discrepancy in age influenced the results (Pashos et al., 2013). In another study, Arden-Close et al. (2011) evaluated the HRQOL of 115 survivors of lymphoma aged 18–45 years for whom it had been more than five years since their last treatment. Men who experienced more late effects from their treatment reported worse psychological HRQOL as measured by the QOL–Cancer Survivor instrument (r = 0.5, p < 0.001). Analysis of variance indicated that although women reported being able to discuss topics of concern, men related that they wanted to review more topics than had been discussed (p = 0.01). These two studies showed varying sex-based differences in anxiety and depression; however, they were also conducted at different time points in the disease trajectory, which may have influenced the results. Stauder et al. (2018) measured anxiety and depression of individuals recently diagnosed with lower-risk MDS using the EQ-5D. They reported that women experienced increased problems with anxiety and depression (p < 0.001) (Stauder et al., 2018). The studies by Pashos et al. (2013) and Stauder et al. (2018) indicated that anxiety and depression were reported more frequently among women when they were initially diagnosed with their hematologic malignancies or at the beginning of treatment. In the study by Arden-Close et al. (2011), which evaluated patients more than five years into survivorship, men reported worse psychological HRQOL. It is unclear why these results differ. However, time of measurement in relation to diagnosis and treatment is an important variable to consider for psychological differences reported.
Pain: The results from three studies (Geyer et al., 2017; Immanuel et al., 2019; Pashos et al., 2013) indicate that women diagnosed with hematologic malignancies experience more pain than men with a similar diagnosis. In a study of 2,006 patients diagnosed with MPNs, Geyer et al. (2017) reported that women had particularly high scores for abdominal-related symptoms (p ≤ 0.001). Of the 2,006 participants, 1,089 were female, lending credibility to their findings that abdominal pain is a greater problem for women diagnosed with MPNs. In a study by Immanuel et al. (2019) of 131 participants with a median age of 66 years, men reported fewer symptoms of pain on the EORTC QLQ-C30 and the EQ-5D instruments. Most participants in this study had lymphoma (Immanuel et al., 2019). Similarly, a study by Pashos et al. (2013) found that, of 1,140 patients (710 males and 430 females), more women (45.2%) than men (35.8%) reported problems with pain or discomfort as measured by EQ-5D domain-specific data (p = 0.0077). Additional research is warranted to improve understanding of the trajectory of pain experienced among patients diagnosed with a hematologic malignancy.
Symptom burden: Symptom burden often results when multiple symptoms occur together, which may cause distress that impairs functioning; this is common among patients with cancer (Cleeland, 2007). In addition to symptom burden, hematologic malignancies may also cause symptom clusters. A symptom cluster is defined as a stable group of concurrent symptoms that are related to one another and distinct from other symptom clusters (Kim et al., 2005). Based on this integrative review, women have a greater symptom burden than men (Geyer et al., 2017; Heutte et al., 2009; Kreissl et al., 2020; Pashos et al., 2013). Among patients with MPNs, Geyer et al. (2017) noted that the microvascular symptom cluster dominated the symptom burden experienced by women. Symptoms in this cluster were headache, fatigue, insomnia, concentration difficulties, and dizziness. Pashos et al. (2013) described a higher symptom burden for female patients diagnosed with CLL. These symptoms were pain (p = 0.0077), fatigue (p < 0.0001), anxiety/depression (p = 0.012), and difficulty doing normal activities (p = 0.0015). Both studies enrolled more than 1,000 patients, and each studied only one type of hematologic malignancy—either MPNs (Geyer et al., 2017) or CLL (Pashos et al., 2013). Studies by Heutte et al. (2009) and Kreissl et al. (2020) focused on survivors of Hodgkin lymphoma. Both studies found that women experienced a greater symptom burden than men. Studies focused on only one type of hematologic malignancy may be key to understanding differences in symptom burden between men and women, because hematologic malignancies are heterogeneous. Additional evidence is needed to further explore symptom burden and symptom clusters among the various hematologic malignancies.
Discussion
Clinical and Research Implications Affecting QOL
Although there are many different hematologic malignancies that affect men and women almost equally, an integrative review of the literature around sex-based differences in QOL, symptoms, and symptom burden experienced by patients and survivors has not been previously published. As a result, these differences have not been recognized or treated effectively. This review integrates the results of several studies to identify the evidence from research of how symptoms, symptom burden, and overall QOL differ between men and women. This knowledge may enable healthcare providers to focus on the relevant needs identified in this integrative review. Current studies indicate that women experience hematologic malignancies differently than men and have increased physical symptoms and limitations, including increased pain and symptom burden, when diagnosed and during treatment. Areas of conflicting data about sex-based differences in the experience of hematologic malignancies include overall QOL, fatigue, and psychological effects, with some studies describing similar experiences between sexes. The data are primarily limited to patients with CLL and lymphoma. More studies are needed to identify or confirm disparities in patients with other hematologic malignancies, such as acute myeloid leukemia, chronic myeloid leukemia, and MDS.
Limitations
This integrative review has several limitations. There were several different instruments used to measure QOL and symptoms in survivors of various hematologic malignancies. This limits comparison between the studies. Other limitations included the number of hematologic malignancies studied and the potential confounding variables of cancer stage and type of treatment. Some of the studies controlled for other confounding variables. However, there was not consistency from one study to another, which limits the generalizability of the results.
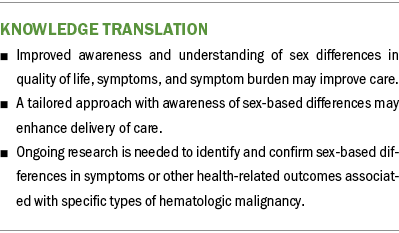
Implications for Nursing
Lower physical functioning in women compared with men has been reported in an evaluation of more than 5,000 cancer survivors (Oertelt-Prigione et al., 2021). These survivors were diagnosed with colorectal, hematologic (e.g., CLL, non-Hodgkin lymphoma, Hodgkin lymphoma, multiple myeloma), basal cell/squamous cell, and thyroid cancers. These findings are similar to those of the current integrative review. With this knowledge, nurses are better prepared to assess and support female patients and survivors. Practice improvements could include enhanced baseline assessments of physical status and customized plans of care developed to assist with identified weaknesses, such as physical therapy consultations. Women may need more conditioning exercises during and following transplantation to maintain physical function and continue independently performing activities of daily living. Other additions to the nursing plan of care may include an expanded pain assessment with the goal of identifying sex-specific interventions to better manage pain.
Conclusions
Despite the limitations, consistent findings were identified for sex-based differences in specific symptoms of hematologic malignancies. Women diagnosed with hematologic malignancies experience a greater physical effect from their cancer and treatment, experience more pain, and carry a greater symptom burden. Improved understanding of sex-based differences in symptoms and treatment side effects in patients with hematologic malignancies may lead to better patient outcomes, and tailored support for individual, sex-specific needs can improve QOL, improve symptom management, and decrease symptom burden.
About the Authors
Sara M. Tinsley-Vance, PhD, APRN, AOCN®, is a nurse practitioner and quality-of-life researcher, Dorothie S. Durosier Mertilus, DNP, APRN, AGNP-C, is a nurse practitioner, and Lisa Nodzon, PhD, APRN, AOCNP®, is an advanced registered nurse practitioner and lead advanced practitioner, all in the Department of Malignant Hematology at the H. Lee Moffit Cancer Center and Research Institute in Tampa, FL; and Cecile A. Lengacher, PhD, RN, FAAN, FAPOS, is a professor and holder of the Lyall and Beatrice Thompson Nursing Professorship in Oncology in the College of Nursing at the University of South Florida in Tampa. This research was supported, in part, by grants from the National Institutes of Health (K23NR018488), the National Institute of Nursing Research, and the Gulf Coast Community Foundation. Writing and editorial support was provided by Paul Fletcher, PhD, and Daley White, BA, research scientific editors at the H. Lee Moffitt Cancer Center Office of Scientific Writing. Tinsley-Vance has previously consulted for AbbVie, Bristol Myers Squibb, Celgene, Incyte, Jazz Pharmaceuticals, and Taiho, and has served on speakers bureaus for Astellas Pharma, Bristol Myers Squibb, Celgene, Incyte, Jazz Pharmaceuticals, Novartis Pharmaceuticals, and Takeda Oncology. Tinsley-Vance and Lengacher contributed to the conceptualization and design and provided the analysis. Tinsley-Vance and Nodzon completed the data collection. All authors contributed to the manuscript preparation. Tinsley-Vance can be reached at sara.tinsleyvance@moffitt.org, with copy to editor at ONFEditor@ons.org. (Submitted March 2022. Accepted December 9, 2022.)
References
Arden-Close, E., Absolom, K., Greenfield, D.M., Hancock, B.W., Coleman, R.E., & Eiser, C. (2011). Gender differences in self-reported late effects, quality of life and satisfaction with clinic in survivors of lymphoma. Psycho-Oncology, 20(11), 1202–1210. https://doi.org/10.1002/pon.1835
Cleeland, C.S. (2007). Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. Journal of the National Cancer Institute Monographs, 2007(37), 16–21. https://doi.org/10.1093/jncimonographs/lgm005
Clinkenbeard, P.R. (1991). Beyond summary: Constructing a review of the literature. In N.K. Buchanan & J.F. Feldhusen (Eds.), Conducting research and evaluation in gifted education: A handbook of methods and applications (pp. 33–50). Teachers College Press.
Critical Appraisal Skills Programme. (2022). CASP systematic review checklist. https://casp-uk.net/casp-tools-checklists
Geyer, H.L., Kosiorek, H., Dueck, A.C., Scherber, R., Slot, S., Zweegman, S., . . . Mesa, R.A. (2017). Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: An analysis by the MPN QOL international working group. Haematologica, 102(1), 85–93. https://doi.org/10.3324/haematol.2016.149559
Heutte, N., Flechtner, H.H., Mounier, N., Mellink, W.A.M., Meerwaldt, J.H., Eghbali, H., . . . Henry-Amar, M. (2009). Quality of life after successful treatment of early-stage Hodgkin’s lymphoma: 10-year follow-up of the EORTC-GELA H8 randomised controlled trial. Lancet Oncology, 10(12), 1160–1170. https://doi.org/10.1016/S1470-2045(09)70258-X
Hofer, F., Koinig, K.A., Nagl, L., Borjan, B., & Stauder, R. (2018). Fatigue at baseline is associated with geriatric impairments and represents an adverse prognostic factor in older patients with a hematological malignancy. Annals of Hematology, 97(11), 2235–2243. https://doi.org/10.1007/s00277-018-3420-8
Immanuel, A., Hunt, J., McCarthy, H., van Teijlingen, E., & Sheppard, Z.A. (2019). Quality of life in survivors of adult haematological malignancy. European Journal of Cancer Care, 28(4), e13067. https://doi.org/10.1111/ecc.13067
Kim, H.-J., McGuire, D.B., Tulman, L., & Barsevick, A.M. (2005). Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nursing, 28(4), 270–282. https://doi.org/10.1097/00002820-200507000-00005
Klimm, B., Reineke, T., Haverkamp, H., Behringer, K., Eich, H.T., Josting, A., . . . Engert, A. (2005). Role of hematotoxicity and sex in patients with Hodgkin’s lymphoma: An analysis from the German Hodgkin study group. Journal of Clinical Oncology, 23(31), 8003–8011. https://doi.org/10.1200/JCO.2005.205.60
Kreissl, S., Müller, H., Goergen, H., Meissner, J., Topp, M., Sökler, M., . . . Borchmann, P. (2020). Health-related quality of life in patients with Hodgkin lymphoma: A longitudinal analysis of the German Hodgkin study group. Journal of Clinical Oncology, 38(25), 2839–2848. https://doi.org/10.1200/JCO.19.03160
Leak, A., Smith, S.K., Crandell, J., Jenerette, C., Bailey, D.E., Zimmerman, S., & Mayer, D.K. (2013). Demographic and disease characteristics associated with non-Hodgkin lymphoma survivors’ quality of life: Does age matter? Oncology Nursing Forum, 40(2), 157–162. https://doi.org/10.1188/13.ONF.157-162
Molica, S. (2006). Sex differences in incidence and outcome of chronic lymphocytic leukemia patients. Leukemia and Lymphoma, 47(8), 1477–1480. https://doi.org/10.1080/10428190600555819
Morishita, S., Kaida, K., Yamauchi, S., Wakasugi, T., Yoshihara, S., Taniguchi, K., . . . Domen, K. (2013). Gender differences in health-related quality of life, physical function and psychological status among patients in the early phase following allogeneic haematopoietic stem cell transplantation. Psycho-Oncology, 22(5), 1159–1166. https://doi.org/10.1002/pon.3128
Mulrow, C., Langhorne, P., & Grimshaw, J. (1997). Integrating heterogeneous pieces of evidence in systematic reviews. Annals of Internal Medicine, 127(11), 989–995. https://doi.org/10.7326/0003-4819-127-11-199712010-00008
Oertelt-Prigione, S., de Rooij, B.H., Mols, F., Oerlemans, S., Husson, O., Schoormans, D., . . . van de Poll-Franse, L.V. (2021). Sex-differences in symptoms and functioning in >5000 cancer survivors: Results from the PROFILES registry. European Journal of Cancer, 156, 24–34. https://doi.org/10.1016/j.ejca.2021.07.019
Pashos, C.L., Flowers, C.R., Kay, N.E., Weiss, M., Lamanna, N., Farber, C., . . . Khan, Z.M. (2013). Association of health-related quality of life with gender in patients with B-cell chronic lymphocytic leukemia. Supportive Care in Cancer, 21(10), 2853–2860. https://doi.org/10.1007/s00520-013-1854-z
Pfreundschuh, M., Müller, C., Zeynalova, S., Kuhnt, E., Wiesen, M.H.J., Held, G., . . . Murawski, N. (2014). Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood, 123(5), 640–646. https://doi.org/10.1182/blood-2013-07-517037
Sarker, S.-J., Smith, S.K., Chowdhury, K., Ganz, P.A., Zimmerman, S., Gribben, J., & Korszun, A. (2017). Comparison of the impact of cancer between British and US long-term non-Hodgkin lymphoma survivors. Supportive Care in Cancer, 25(3), 739–748. https://doi.org/10.1007/s00520-016-3454-1
Schmetzer, O., & Flörcken, A. (2012). Sex differences in the drug therapy for oncologic diseases. Handbook of Experimental Pharmacology, 214, 411–442. https://doi.org/10.1007/978-3-642-30726-3_19
Seow, H., Barbera, L., Sutradhar, R., Howell, D., Dudgeon, D., Atzema, C., . . . Earle, C. (2011). Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. Journal of Clinical Oncology, 29(9), 1151–1158. https://doi.org/10.1200/jco.2010.30.7173
Shanafelt, T.D., Bowen, D., Venkat, C., Slager, S.L., Zent, C.S., Kay, N.E., . . . Call, T.G. (2007). Quality of life in chronic lymphocytic leukemia: An international survey of 1482 patients. British Journal of Haematology, 139(2), 255–264. https://doi.org/10.1111/j.1365-2141.2007.06791.x
Siegel, R.L., Miller, K.D., Wagel, N.S., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48. https://doi.org/10.3322/caac.21763
Stauder, R., Yu, G., Koinig, K.A., Bagguley, T., Fenaux, P., Symeonidis, A., . . . de Witte, T. (2018). Health-related quality of life in lower-risk MDS patients compared with age- and sex-matched reference populations: A European LeukemiaNet study. Leukemia, 32(6), 1380–1392. https://doi.org/10.1038/s41375-018-0089-x



