Domains of Structured Oral Anticancer Medication Programs: A Scoping Review
Problem Identification: An interprofessional approach is necessary to support the multifactorial process of patient adherence to oral anticancer medications (OAMs). This scoping review aims to identify structured OAM programs in published literature, identify components within studies, and propose a framework for institutions developing or maintaining OAM programs.
Literature Search: Embase®, PubMed®, and CINAHL® databases were searched for studies published between January 2000 and April 2021.
Data Evaluation: Two reviewers screened studies and extracted data. Characteristics and specific domains of the OAM programs were captured. Key components of the programs were identified, and a framework was created to guide program development.
Synthesis: Components identified among the 21 studies were education; counseling; follow-up; dedicated clinician contact; adverse event and toxicity monitoring; adherence monitoring; drug procurement, delivery, and supply; patient- and system-level cost reduction; information technology; and risk assessment.
Implications for Research: Based on the findings, a framework for building and evaluating OAM adherence programs is proposed. Future studies should evaluate the reliability and validity of this framework because further testing may lead to the development of additional components.
Jump to a section
With increased use of oral anticancer medications (OAMs) (Bedell, 2003), oncology programs need to adapt their processes to support patients who are self-administering oral cancer treatment at home instead of delivery via IV at an infusion center. Patients who are taking OAMs at home must obtain and correctly schedule and dose their medications, monitor for toxicity, and know what to do when questions or issues arise. Clinical sites need processes to support patients in these efforts, which may include prescribing and obtaining medications, educating, and monitoring for OAM adherence and side effects (Tipton, 2015). Safety standards and practice guidelines (Mackler et al., 2018; Neuss et al., 2013) have helped to address these new challenges, but continued work is needed. Medication adherence, defined as the process of taking one’s medications as prescribed, is complex and includes the following phases that are interrelated yet distinct: initiation (taking the first prescribed dose), implementation (the extent to which actual dosing corresponds to the prescribed regimen), discontinuation (cessation of taking the prescribed medication, for whatever reason), and persistence (the time between initiation and discontinuation) (De Geest et al., 2018; Vrijens et al., 2012). The association between OAM adherence and patient outcomes has been documented (Gupta et al., 2018; Jacobs et al., 2019; Jiang et al., 2020). Because of the complexity of OAM adherence and connectedness to patient outcomes, it is critical to use an interprofessional, multifactorial approach to support patients and improve OAM adherence throughout the treatment trajectory.
Studies have reported OAM programs and their benefits to patients; however, these programs each vary regarding roles and responsibilities of clinicians involved, as well as what interventions are included in the program. Previous reviews have investigated various interventions that may improve OAM adherence in patients with cancer (Kavookjian & Wittayanukorn, 2015; Krikorian et al., 2019; Zerillo et al., 2018), but reviews assessing the specific components of OAM programs and their effects on adherence are limited. Passey et al. (2020) conducted a systematic review of pharmacist-led medication management programs for oral antineoplastic therapy and reported that all programs had one or more positive outcomes, including improved adherence, cost savings, and patient satisfaction. The programs reviewed included standardized documentation templates, workflow changes, patient education and counseling, adverse event monitoring, and dose modifications (Passey et al., 2020).
The objective of this scoping review was to identify structured OAM programs described in the literature, compare components within the programs, and propose a framework for institutions to use and reference to support the development and maintenance of OAM adherence programs.
Methods
This scoping review was conducted using four main steps. First, the search strategy and eligibility criteria were designed, and searches were run in the relevant databases. Second, titles and abstracts of the identified articles were screened, followed by full-text screening. Third, relevant data were extracted from the included studies into a standardized and pilot-tested form. Lastly, through content analysis and iterative review with clinical experts, a narrative synthesis was developed, as well as a framework for institutions wanting to implement an OAM program or as a benchmark for current programs. This scoping review followed guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews (Tricco et al., 2018).
This review included collaboration with topic-specific experts to tailor and contextualize the review and findings. The clinical experts included experienced oncology nurses from the Oncology Nursing Society with clinical backgrounds and experience working with patients receiving OAMs.
Search Strategy and Screening
The search strategy was designed to identify studies that discussed structured OAM programs and their impact on OAM adherence. An information specialist developed searches in Embase®, PubMed®, and CINAHL® from January 1, 2000, through April 30, 2021, for English-language studies. The start date for the search was chosen to correspond with the increase in OAM use in clinical care. Full search strategies are presented in the appendices.
Eligibility criteria included English-language studies with institution- or practice-level OAM programs and discussion of the effect these programs had on adherence. Letters to the editor, commentaries, and editorials were excluded. Systematic reviews were included at the title and abstract review stage to retrieve the full text and review references lists for any other relevant studies on the study topic.
Two reviewers independently and in duplicate screened titles and abstracts to assess eligibility. Disagreements were discussed and resolved by consensus with a third reviewer. If initial screening indicated an OAM program with discussion of adherence, the full-text article was retrieved and reviewed. Next, full-text articles were screened independently by two reviewers, and disagreements were resolved by consensus with a third reviewer adjudicating. All screening was conducted using Covidence software.
Data Extraction and Analysis
Data were extracted from eligible studies into a standardized and pilot-tested Microsoft Excel spreadsheet. Elements captured from each selected study were the location where the study was conducted; study design; study period; funding source; size of program; type of institution delivering the program; title of program; whether the program was based on a previously developed program, grounded in a theoretical framework, and clinic- or pharmacy-based; the primary developer, primary user, and included population (e.g., limited to OAMs or all treatments); program costs and cost to patients; relationship between program and adherence to OAMs; number of components within the program; and the name and description of each component. Other elements captured were whether the program reported on measures of adherence, whether the utility of the program was measured, and whether sustainability or implementation strategies were discussed. If these elements were discussed, descriptions of each were extracted. The objective of the program and the phase of adherence were also captured based on the adherence process phases outlined in the ESPACOMP Medication Adherence Reporting Guideline (De Geest et al., 2018). The phases include initiation (taking the first does of a prescribed medication), implementation (the extent to which actual dosing corresponds to prescribed), and persistence (the time between initiation and last dose) (De Geest et al., 2018).
A narrative synthesis of the literature was produced using the findings extracted from the studies. Components were defined as the broad themes that the programs included, and common components of the structured OAM programs addressing adherence were identified through content analysis. First, components of OAM programs were identified because they were distinctly categorized in the studies (e.g., education). Based on this organization, a comprehensive framework was further developed with all components identified across the programs. Finally, following an iterative review with clinical experts, a framework was proposed for institutions wanting to implement and/or evaluate an existing OAM program.
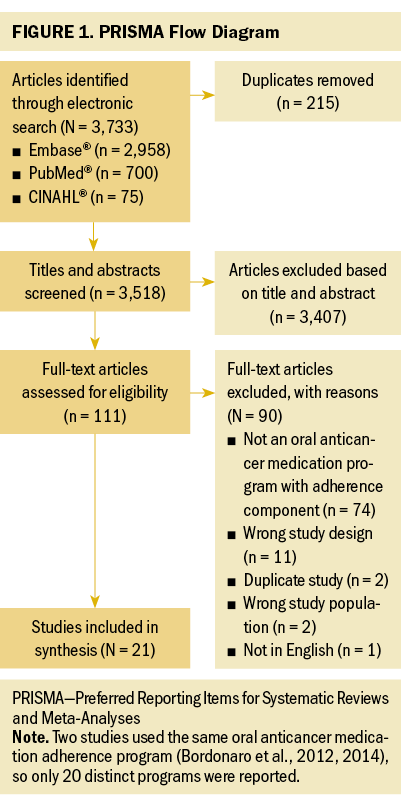
Results
The search yielded 3,733 articles, of which 215 were duplicates (see Figure 1). Of the remaining 3,518, 111 articles met eligibility criteria based on title and abstract screening. After full-text screening, 21 studies reporting on distinct programs were included (Battis et al., 2017; Bordonaro et al., 2012, 2014; Conliffe et al., 2019; Curry et al., 2020; Dennison et al., 2021; Deutsch et al., 2016; Gebbia et al., 2013; Jean et al., 2016; Khandelwal et al., 2012; Krolop et al., 2013; Lam & Cheung, 2016; Middendorff et al., 2018; Moreira et al., 2019; Morgan et al., 2018; Muluneh et al., 2018; Ribed et al., 2016; Riu et al., 2018; Tschida et al., 2012; Vacher et al., 2020; Wong et al., 2014). Most studies were conducted in the United States (n = 12), with the remainder conducted in Italy (n = 3), France (n = 2), Spain (n = 2), Germany (n = 1), and Brazil (n = 1). All but one of the studies were observational cohort studies. Most of the OAM adherence programs were pharmacy- (n = 10) or clinic-based (n = 8), with the remaining being other (e.g., home care) (n = 2) or unspecified (n = 1). Most programs’ approaches targeted the implementation phase of OAM therapy (n = 14), whereas others focused on both initiation and implementation (n = 6), and one targeted the initiation phase. Specific study characteristics and program components are presented in the appendices.
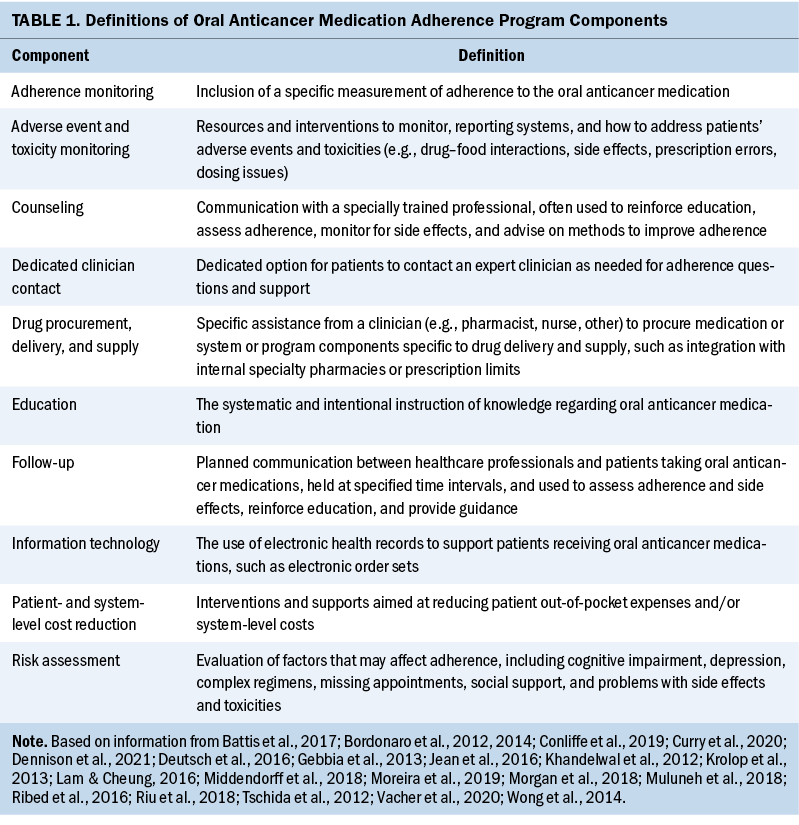
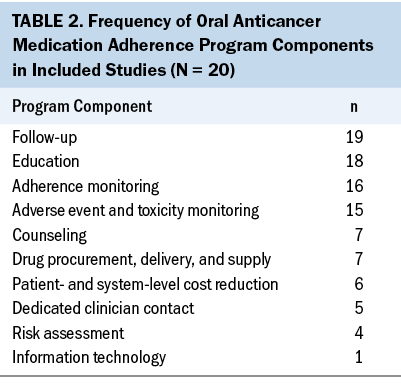
The following 10 distinct components from the included OAM programs were identified: education; counseling; follow-up; dedicated clinician contact; adverse event and toxicity monitoring; adherence monitoring; drug procurement, delivery, and supply; patient- and system-level cost reduction; information technology; and risk assessment. A definition was developed for each component by extracting and synthesizing the descriptions of the interventions described in the studies (see Table 1). Follow-up, education, adherence monitoring, and adverse event and toxicity monitoring were identified in 70% (n = 14) of the programs, whereas information technology was identified in only one program (see Table 2).
Education
For this review, education was defined as the systematic and intentional instruction of knowledge regarding OAMs. Of the 20 programs identified, 18 distinctly reported on education as a component of the OAM program addressing adherence. The programs were within a clinical or pharmacy setting, and all focused on the implementation phase of adherence, with six also focused on initiation. The reported educational content included information about the patient’s disease state; medication purpose, mechanism of action, and administration; potential side effects and their management; drug handling and storage; drug interactions; adherence; and follow-up testing and appointments. Several studies cited the use of verbal and written education and holding the sessions face-to-face or in a virtual setting prior to treatment initiation. Education was provided by a chemotherapy-competent pharmacist or nurse when reported. Two programs held additional educational sessions for individuals identified as nonadherent following an initial assessment (Krolop et al., 2013; Vacher et al., 2020).
Counseling
For this review, counseling was defined as communication with a specially trained professional, often used to reinforce education, assess adherence, monitor for side effects, and advise on methods to improve adherence. Of the programs, seven distinctly reported on counseling as a component of the OAM program addressing adherence. The programs were within a clinic- or pharmacy-based setting, and all focused on the implementation phase of adherence, with two also focused on initiation. Counseling with a specially trained professional was used to reinforce education, assess adherence, monitor for and assist with treatment of side effects, and provide advice on methods to improve adherence to OAMs. Counseling took place in face-to-face sessions in-person, virtually, or via telephone. Patients were also provided with supportive information and education.
Follow-Up
Follow-up was defined as planned communication between healthcare professionals and patients receiving OAMs that was held at specified time intervals and used to assess adherence and side effects, reinforce education, and provide guidance. Follow-up was distinctly reported as a component of the OAM program in 19 of the programs identified. The programs were within homecare, clinic-, or pharmacy-based settings, and all focused on the implementation phase of adherence, with four also focused on initiation. Follow-up was planned, and proactive communication between healthcare professionals and patients receiving OAMs was used to assess adherence and side effects. It was an opportunity to provide counseling and reinforce education. Follow-up was held at time intervals specific to the program, the drug, or the patient and occurred weekly to monthly.
Dedicated Clinician Contact
Dedicated clinician contact was defined as having a dedicated option for patients to contact an expert clinician as needed for adherence questions and support. Of the 20 programs, 5 distinctly reported on expert contact as a component of the OAM program addressing adherence. The programs were within homecare, clinic-, or pharmacy-based settings. Each program focused on the implementation phase of adherence, with two also focused on initiation. The different ways the programs provided expert contact included a dedicated telephone line and fast-track visit system, as well as 24/7 access to reach a clinician by telephone with any questions regarding treatment or side effects.
Adverse Event and Toxicity Monitoring
Adverse event and toxicity monitoring was defined as resources and interventions to monitor, report, and address patients’ adverse events and toxicities. Of the studies reviewed, 15 distinctly reported on adverse event and toxicity monitoring as a component of the OAM program addressing adherence. Most of these patient encounters occurred either in the clinic or remotely, although one German program was uniquely home-based (Bordonaro et al., 2012, 2014). Each program focused on the implementation phase of adherence, with six also focused on initiation. Having trained personnel was important in most of these programs, and extra effort was sometimes allocated to allow dedicated individuals to function in these roles. Some personnel and/or staff members were able to intervene and manage toxicities directly (e.g., clinical pharmacist practitioners with collaborative practice agreements [Muluneh et al., 2018]), whereas others served as a liaison between patients and providers. One program also included 24/7 dedicated clinical support call lines (Middendorff et al., 2018). Another program documented activities in the electronic health record (Wong et al., 2014). Some programs included standardized or prespecified patient education and follow-up intervals for adverse event and toxicity assessment, but time intervals differed across studies. Conliffe et al. (2019) completed monitoring and testing specific to each chemotherapy regimen and included preprinted chemotherapy orders that included U.S. Food and Drug Administration–approved labeled monitoring recommendations, which aligned with the American Society of Clinical Oncology/Oncology Nursing Society monitoring and safety standards. Deutsch et al. (2016) reported on standardized counseling points for each medication, with each potential adverse event built into their assessment. Other programs included individualized follow-up visits based on patients’ risk categories, including patient categorization of stable versus unstable (Dennison et al., 2021) and participants who were on medications known to have high discontinuation rates (defined as more than 20%) (Deutsch et al., 2016). One program (Gebbia et al., 2013) also included patients’ caregivers in this assessment. Three programs used established toxicity grading criteria, using the Eastern Cooperative Oncology Group (Bordonaro et al., 2012) and the National Cancer Institute Common Terminology Criteria for Adverse Events (Gebbia et al., 2013; Khandelwal et al., 2012).
Adherence Monitoring
Adherence monitoring was defined as the inclusion of a specific measurement of adherence to the OAM. Of the programs identified, 16 distinctly reported on adherence monitoring and support as a component of the OAM program. Programs were based in the pharmacy, clinic, or home setting and involved the implementation or initiation and implementation phases of adherence. The programs included adherence monitoring by questionnaire or self-report (n = 5), medication possession ratio (n = 5), the Morisky Medication Adherence Scale (n = 1) (Morisky et al., 1986), the Basel Assessment of Adherence (n = 1) (Gebbia et al., 2013), and AARDEX’s Electronic Medication Monitoring Systems (n = 1), which is a formula, observation, or an unclear description of adherence.
Drug Procurement, Delivery, and Supply
This component was defined as specific assistance from a clinician (e.g., pharmacist, nurse, other personnel) to procure medication or program components specific to drug delivery and supply, such as integration with internal specialty pharmacies or prescription limits. Of the programs identified, seven distinctly reported on drug procurement, delivery, and supply as a component of the OAM addressing adherence. The programs were a mix of pharmacy- (n = 4) and clinic-based (n = 3). Programs included patients during the initiation and implementation phases of adherence. The programs provided drug procurement, delivery, and supply in a variety of ways, including limiting supply to allow assessment of patient tolerance and safety, drug procurement assistance (e.g., prior authorizations, insurance assistance), notification of pharmacy support services for uninsured patients, partial supply dispense, assistance with medication access, and review and confirmation of comprehensive lists of concurrent medications.
Patient- and System-Level Cost Reduction
This component was defined as interventions and supports aimed at reducing patient out-of-pocket expenses and/or system-level costs. Of the programs identified, six distinctly reported on cost reduction at the patient- and/or system-level as a component of the OAM program addressing adherence. The programs focused on the implementation phase of adherence, with three also focusing on initiation. Three programs addressed patients’ out-of-pocket expenses (e.g., insurance evaluation, assistance with prior authorizations and co-pays, split-fill contracts with eligible payers to supply the first half of the month’s therapy at half of the patient’s co-pay, manufacturer- and disease-specific program assistance); the team members handling this work varied and included patient advocates, case managers, and trained and dedicated program personnel (Deutsch et al., 2016; Middendorff et al., 2018; Muluneh et al., 2018). Programs also evaluated their effectiveness at system-level cost savings. Two programs reported that improvements to patient adherence had reduced costs associated with medication use and patient care (Tschida et al., 2012; Wong et al., 2014). Another program reported that an on-site pharmacy dispensing program had yielded a net profit, but only two initial fills had occurred on-site because of the requirement of most insurance companies to fill prescriptions via mail-order pharmacies (Conliffe et al., 2019).
Information Technology
Information technology was defined as the use of electronic health records to support patients receiving OAMs, such as electronic order sets. Of the programs, one distinctly reported on information technology as a component of the OAM program addressing adherence. This program was pharmacy-based and focused on the implementation phase of adherence (Battis et al., 2017). The program created drug-specific electronic order sets to prompt providers to prescribe appropriate medications and pertinent labs for monitoring.
Risk Assessment
Risk assessment was broadly defined as evaluating factors that may affect adherence (e.g., cognitive impairment, depression, complex regimens, missed appointments, social support, side effects or toxicities). Of the programs identified, four distinctly reported on risk assessment as a domain of OAM program adherence. The programs were a mix of pharmacy- and clinic-based and focused on the implementation phase of adherence. One study (Battis et al., 2017) conducted patient risk assessments evaluating factors that correlated with adherence but did not describe the assessment in detail. Two programs (Krolop et al., 2013; Vacher et al., 2020) targeted the implementation phase of adherence by screening patients for low levels of adherence at therapy initiation and then provided targeted support via tailored education and follow-up. Another study used personnel and a dedicated clinic to provide in-person and telephone encounters to proactively monitor and address midcycle toxicities (Curry et al., 2020). The researchers noted that these dedicated patient encounters and staff members seemed to foster patients’ comfort with communicating toxicity concerns outside of scheduled times.
OAM Program Framework
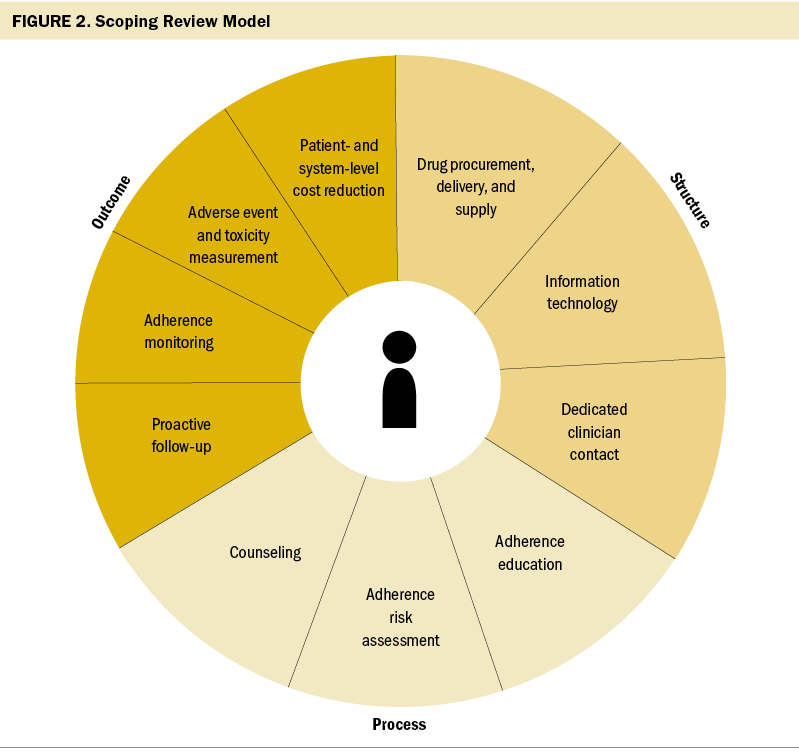
The identified components were grouped into the structure, process, and outcomes model first identified by Donabedian (1966) as a tool to evaluate healthcare quality (Ayanian & Markel, 2016). Structure is defined as the settings, provider qualifications, and administrative systems where care takes place; in the current framework, structures are delivery and supply of medications, information technology, and a process to allow for dedicated clinician contact. Process is described as the components of care delivered; in the current framework, these are adherence education, risk assessment, counseling, and proactive follow-up. Outcomes are recovery, restoration of function, and survival, which are measured in the current framework using adherence monitoring, adverse event and toxicity monitoring, and patient- and system-level cost reductions. The proposed framework to describe program components to improve OAM adherence are visualized in Figure 2.
Discussion
This scoping review identified 20 distinct OAM programs reported in the literature, which included an aggregate of 10 components aimed at improving patient adherence. The programs included heterogeneous populations of patients at different phases of adherence. The main components used by the programs included education; counseling; follow-up; dedicated clinician contact; adverse event and toxicity monitoring; adherence monitoring and support; drug procurement, delivery, and supply; patient- and system-level cost reduction; information technology; and risk assessment. Based on the findings, a framework is proposed that includes important components to consider when creating or evaluating an existing OAM program. Although a comprehensive framework of components presented in the literature is presented, which components are most crucial to optimal adherence are unknown. The final proposed framework aimed to solidify program components represented in the literature and present options for institutions to consider when developing and/or evaluating OAM programs.
Strengths and Weaknesses
This review has several strengths. This review was conducted following guidance for scoping reviews, with transparency and the appropriate methodology. Screening followed rigorous systematic review methodology, and collating, summarizing, and reporting results followed the scoping review guidance from PRISMA (Tricco et al., 2008). An informative, iterative process was used for synthesizing relevant evidence and constructing the findings into a framework to guide the development of future OAM programs and the evaluation or maintenance of existing programs. In addition, the review allowed for scoping the literature pertaining to OAM programs aimed at improving adherence and identifying research and practice gaps in need of additional consideration.
However, there are limitations to this study. The development of the final table for the guidance of OAM programs was iteratively developed by a team of nurses, researchers, and methodologists, and replicability is not guaranteed. Because of the nature of scoping reviews, biases are possible due to the lack of quality assessment of the included studies. The effectiveness of the OAM programs represented by this body of literature has not been evaluated. Frequencies of presentation of items were also not used to determine what should be included in the final framework. The authors recommend that future research test and elucidate mechanisms of identified components, as well as test the validity of the proposed framework.
Relation to Other Studies and Guidelines
Various systematic reviews have explored the relationship between OAM and patient adherence, as well as interventions to improve adherence (Kavookjian & Wittayanukorn, 2015; Krikorian et al., 2019; Zerillo et al., 2018), and several studies have investigated OAM programs and their effects on OAM adherence (Morgan et al., 2018; Muluneh et al., 2018; Patel et al., 2016). However, the current review explores specific components across OAM programs in the literature aiming to improve adherence. Rosenberg et al. (2020) conducted a scoping literature review exploring interventions that improved adherence to OAMs; however, this review evaluated specific interventions and not OAM programs. The Oncology Nursing Society and American Society of Clinical Oncology standards for oral chemotherapy administration (Neuss et al., 2013, 2017) recommend completing a risk assessment for patients who are starting an OAM regimen. Despite these recommendations, the authors’ review of the literature identified few examples of programs that had implemented this recommendation into practice specific to OAM therapies, an important gap that needs to be addressed in this changing paradigm of cancer therapy administration.
Implications for Nursing
This scoping review provides a comprehensive framework to guide clinicians and institutions wanting to implement and/or evaluate programs aimed at improving OAM adherence. Programs and policies to support patients receiving OAMs are important and should be comprehensive, addressing components important to stakeholders, such as patients, clinicians, and administrators. Identifying the components of reported programs may assist clinical sites with operationalizing OAM program processes in ways that are more likely to improve patient adherence to OAMs.

Conclusion
Gaps remain in the OAM adherence program literature, and the implementation and sustainability of individual programs has not been well described. In addition, how studies operationalize and measure adherence to OAMs varies. Defining complementary interprofessional roles and responsibilities of clinicians involved in these programs, as well as what programmatic interventions are most effective during specific phases of adherence, remains an important area for ongoing dialogue and inquiry. Next steps would be to evaluate the reliability and validity of this framework for use in clinical practice. Further testing of and expansion on this framework as the OAM literature evolves might lead to the development of additional components or methods and strategies for implementation.
About the Authors
Kapeena Sivakumaran, MPH, is a research assistant at the Evidence Foundation in Cleveland Heights, OH; Pamela K. Ginex, EdD, MPH, RN, OCN®, is an assistant professor in the School of Nursing at Stony Brook University with a joint appointment in the Division of Population Science at the Stony Brook Cancer Center, both in New York, and was, at the time of writing, the senior manager of evidence-based practice and inquiry at the Oncology Nursing Society in Pittsburgh, PA; Haya Waseem, MPH, is a research assistant at the Evidence Foundation; Sarah M. Belcher, PhD, RN, OCN®, is an assistant professor in the School of Nursing and the Palliative Research Center at the University of Pittsburgh in Pennsylvania; Sarah Lagler-Clark, MPH, is a practicum student at the Evidence Foundation and a graduate research assistant in the Department of Health Research Methods, Evidence and Impact at McMaster University in Hamilton, Ontario, Canada; Kristine B. LeFebvre, MSN, RN, NPD-BC, AOCN®, is an oncology clinical specialist at the Oncology Nursing Society; Nicole Palmer, MPH, is a research assistant at the Evidence Foundation; Tejanth Pasumarthi, is a research student at the Evidence Foundation and a student in the School of Interdisciplinary Science at McMaster University; and Rebecca L. Morgan, PhD, MPH, is the executive director at the Evidence Foundation and an assistant professor in the Department of Health Research Methods, Evidence and Impact at McMaster University. Development of this review was wholly funded by the Oncology Nursing Society, a nonprofit organization that represents oncology nurses. Belcher’s scholarship is supported, in part, by the National Institute of Nursing Research (K23NR019296). Sivakumaran, Ginex, and Morgan contributed to the conceptualization and design. Sivakumaran, Ginex, Belcher, Lagler-Clark, LeFebvre, Palmer, Pasumarthi, and Morgan completed the data collection. Sivakumaran, Palmer, and Morgan provided statistical support. Sivakumaran, Ginex, Belcher, LeFebvre, and Morgan provided the analysis. Sivakumaran, Ginex, Waseem, Belcher, LeFebvre, Palmer, Pasumarthi, and Morgan contributed to the manuscript preparation. Ginex can be reached at pamela.ginex@stonybrook medicine.edu, with copy to ONFEditor@ons.org. (Submitted November 2021. Accepted January 15, 2022.)
References
Ayanian, J.Z., & Markel, H. (2016). Donabedian’s lasting framework for health care quality. New England Journal of Medicine, 375(3), 205–207. https://doi.org/10.1056/NEJMp1605101
Battis, B., Clifford, L., Huq, M., Pejoro, E., & Mambourg, S. (2017). The impacts of a pharmacist-managed outpatient clinic and chemotherapy-directed electronic order sets for monitoring oral chemotherapy. Journal of Oncology Pharmacy Practice, 23(8), 582–590. https://doi.org/10.1177/1078155216672314
Bedell, C.H. (2003). A changing paradigm for cancer treatment: The advent of new oral chemotherapy agents. Clinical Journal of Oncology Nursing, 7(6, Suppl.), 5–9. https://doi.org/10.1188/03.CJON.s6.5-9
Bordonaro, S., Raiti, F., Di Mari, A., Lopiano, C., Romano, F., Pumo, V., . . . Tralongo, P. (2012). Active home-based cancer treatment. Journal of Multidisciplinary Healthcare, 2012(5), 137–143. https://doi.org/10.2147/jmdh.S31494
Bordonaro, S., Romano, F., Lanteri, E., Cappuccio, F., Indorato, R., Butera, A., . . . Tralongo, P. (2014). Effect of a structured, active, home-based cancer-treatment program for the management of patients on oral chemotherapy. Patient Preference and Adherence, 2014(8), 917–923. https://doi.org/10.2147/ppa.S62666
Conliffe, B., Figg, L., Moffett, P., Lauterwasser, L., & Parsons, L.B. (2019). Impact of a formal pharmacist-run oral antineoplastic monitoring program: A pilot study in an adult genitourinary oncology clinic. Journal of Oncology Pharmacy Practice, 25(4), 777–786. https://doi.org/10.1177/1078155217753889
Curry, M.A., Chineke, I., Redelico, T., Terrell, C., Bell, W., Flood, D., . . . Bernal-Mizrachi, L. (2020). Adherence to oral anticancer medications after implementation of an ambulatory adherence program at a large urban academic hospital. JCO Oncology Practice, 16(4), e350–e356. https://doi.org/10.1200/jop.19.00167
De Geest, S., Zullig, L.L., Dunbar-Jacob, J., Helmy, R., Hughes, D.A., Wilson, I.B., & Vrijens, B. (2018). ESPACOMP medication adherence reporting guideline (EMERGE). Annals of Internal Medicine, 169(1), 30–35. https://doi.org/10.7326/m18-0543
Dennison, T., Deal, A.M., Foster, M., Valgus, J., & Muluneh, B. (2021). A pharmacist-led oral chemotherapy program’s impact on chronic myeloid leukemia patient satisfaction, adherence, and outcomes. Journal of the Advanced Practitioner in Oncology, 12(2), 148–157. https://doi.org/10.6004/jadpro.2021.12.2.3
Deutsch, S., Koerner, P., Miller, R.T., Craft, Z., & Fancher, K. (2016). Utilization patterns for oral oncology medications in a specialty pharmacy cycle management program. Journal of Oncology Pharmacy Practice, 22(1), 68–75. https://doi.org/10.1177/1078155214547664
Donabedian, A. (1966). Evaluating the quality of medical care. Milbank Memorial Fund Quarterly, 44(3), 166–206. https://doi.org/10.2307/3348969
Gebbia, V., Bellavia, M., Banna, G.L., Russo, P., Ferraù, F., Tralongo, P., & Borsellino, N. (2013). Treatment monitoring program for implementation of adherence to second-line erlotinib for advanced non-small-cell lung cancer. Clinical Lung Cancer, 14(4), 390–398. https://doi.org/10.1016/j.cllc.2012.11.007
Gupta, S., Abouzaid, S., Liebert, R., Parikh, K., Ung, B., & Rosenberg, A.S. (2018). Assessing the effect of adherence on patient-reported outcomes and out of pocket costs among patients with multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia, 18(3), 210–218. https://doi.org/10.1016/j.clml.2018.01.006
Jacobs, J.M., Ream, M.E., Pensak, N., Nisotel, L.E., Fishbein, J.N., MacDonald, J.J., . . . Greer, J.A. (2019). Patient experiences with oral chemotherapy: Adherence, symptoms, and quality of life. Journal of the National Comprehensive Cancer Center, 17(3), 221–228. https://doi.org/10.6004/jnccn.2018.7098
Jean, E.P., Etienne-Selloum, N., Regnier, O., Poirot, B., Abdelghani, M.B., & Prébay, D. (2016). Pharmaceutical care consultations and multidisciplinary educational program for improving adherence in oncology. International Journal of Clinical Pharmacy, 38(5), 1343. https://doi.org/10.1007/s11096-016-0347-9
Jiang, Y., Wickersham, K.E., Zhang, X., Barton, D.L., Farris, K.B., Krauss, J.C., & Harris, M.R. (2020). Side effects, self-management activities, and adherence to oral anticancer agents. Patient Preference and Adherence, 2019(13), 2243–2252. https://doi.org/10.2147/ppa.s224496
Kavookjian, J., & Wittayanukorn, S. (2015). Interventions for adherence with oral chemotherapy in hematological malignancies: A systematic review. Research in Social and Administrative Pharmacy, 11(3), 303–314. https://doi.org/10.1016/j.sapharm.2014.08.006
Khandelwal, N., Duncan, I., Ahmed, T., Rubinstein, E., & Pegus, C. (2012). Oral chemotherapy program improves adherence and reduces medication wastage and hospital admissions. Journal of the National Comprehensive Cancer Center, 10(5), 618–625. https://doi.org/10.6004/jnccn.2012.0063
Krikorian, S., Pories, S., Tataronis, G., Caughey, T., Chervinsky, K., Lotz, M., . . . Weissmann, L. (2019). Adherence to oral chemotherapy: Challenges and opportunities. Journal of Oncology Pharmacy Practice, 25(7), 1590–1598. https://doi.org/10.1177/1078155218800384
Krolop, L., Ko, Y.D., Schwindt, P.F., Schumacher, C., Fimmers, R., & Jaehde, U. (2013). Adherence management for patients with cancer taking capecitabine: A prospective two-arm cohort study. BMJ Open, 3(7), e003139. https://doi.org/10.1136/bmjopen-2013-003139
Lam, M.S.H., & Cheung, N. (2016). Impact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemia. Journal of Oncology Pharmacy Practice, 22(6), 741–748. https://doi.org/10.1177/1078155215608523
Mackler, E., Segal, E.M., Muluneh, B., Jeffers, K., & Carmichael, J. (2018). 2018 Hematology/Oncology Pharmacist Association best practice for the management of oral oncolytic therapy: Pharmacy practice standard. Journal of Oncology Practice, 15(4), e364–e355. https://doi.org/10.1200/JOP.18.00581
Middendorff, G., Elsey, R., Lounsbery, B., & Chadwell, R. (2018). Impact of a specialty pharmacy case management service on adherence in patients receiving oral antineoplastic agents. Journal of Oncology Pharmacy Practice, 24(5), 371–378. https://doi.org/10.1177/1078155217708022
Moreira, G., Ferreira, C.G., Montella, T., Vasconcellos, J., & Goncalves, I. (2019). Pharmaceutical follow-up program for patients with oral drug treatment in non-small cell lung cancer in a heterogeneous health care system. Journal of Thoracic Oncology, 14(11, Suppl. 2), S1184. https://doi.org/10.1016/j.jtho.2019.09.161
Morgan, K.P., Muluneh, B., Deal, A.M., & Amerine, L.B. (2018). Impact of an integrated oral chemotherapy program on patient adherence. Journal of Oncology Pharmacy Practice, 24(5), 332–336. https://doi.org/10.1177/1078155217703792
Morisky, D.E., Green, L.W., & Levine, D.M. (1986). Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care, 24(1), 67–74. https://doi.org/10.1097/00005650-198601000-00007
Muluneh, B., Schneider, M., Faso, A., Amerine, L., Daniels, R., Crisp, B., . . . Savage, S. (2018). Improved adherence rates and clinical outcomes of an integrated, closed-loop, pharmacist-led oral chemotherapy management program. Journal of Oncology Practice, 14(6), e324–e334. https://doi.org/10.1200/jop.17.00039
Neuss, M., Gilmore, T., Belderson, K., Billett, A., Conti-Kalchik, T., Harvey, B., . . . Polovich, M. (2017). 2016 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards, including standards for pediatric oncology. Oncology Nursing Forum, 44(1), 31–43. https://doi.org/10.1188/17.ONF.31-43
Neuss, M.N., Polovich, M., McNiff, K., Esper, P., Gilmore, T.R., LeFebvre, K.B., . . . Jacobson, J.O. (2013). 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherpay administration safety standards including standards for the safe administraiton and management of oral chemotherapy. Journal of Oncology Practice, 9(2, Suppl.), 5S–13S. https://doi.org/10.1200/JOP.2013.000874
Passey, D.G., Healy, R., Qualls, J., Halwani, A., & Sauer, B.C. (2020). Pharmacist-led collaborative medication management programs for oral antineoplastic therapies: A systematic literature review. Journal of the American Pharmacists Association, 61(3), e7–e18. https://doi.org/10.1016/j.japh.2020.12.005
Patel, J.M., Holle, L.M., Clement, J.M., Bunz, T., Niemann, C., & Chamberlin, K.W. (2016). Impact of a pharmacist-led oral chemotherapy-monitoring program in patients with metastatic castrate-resistant prostate cancer. Journal of Oncology Pharmacy Practice, 22(6), 777–783. https://doi.org/10.1177/1078155215612541
Ribed, A., Romero-Jiménez, R.M., Escudero-Vilaplana, V., Iglesias-Peinado, I., Herranz-Alonso, A., Codina, C., & Sanjurjo-Sáez, M. (2016). Pharmaceutical care program for onco-hematologic outpatients: Safety, efficiency and patient satisfaction. International Journal of Clinical Pharmacy, 38(2), 280–288. https://doi.org/10.1007/s11096-015-0235-8
Riu, G., Gaba, L., Victoria, I., Molas, G., do Pazo, F., Gómez, B., . . . Vidal, L. (2018). Implementation of a pharmaceutical care programme for patients receiving new molecular-targeted agents in a clinical trial unit. European Journal of Cancer Care, 27(1), e12447. https://doi.org/10.1111/ecc.12447
Rosenberg, S.M., Petrie, K.J., Stanton, A.L., Ngo, L., Finnerty, E., & Partridge, A.H. (2020). Interventions to enhance adherence to oral antineoplastic agents: A scoping review. Journal of the National Cancer Institute, 112(5), 443–465. https://doi.org/10.1093/jnci/djz244
Tipton, J. (2015). Overview of the challenges related to oral agents for cancer and their impact on adherence. Clinical Journal of Oncology Nursing, 19(3, Suppl.), 37–40. https://doi.org/10.1188/15.S1.CJON.37-40
Tricco, A.C., Lillie, E., Zarin, W., O’Brien, K.K., Colquhoun, H., Levac, D., . . . Straus, S.E. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. https://doi.org/10.7326/m18-0850
Tschida, S.J., Aslam, S., Lal, L.S., Khan, T.T., Shrank, W.H., Bhattarai, G.R., . . . Sahli, B.D. (2012). Outcomes of a specialty pharmacy program for oral oncology medications. American Journal of Pharmacy Benefits, 4(4), 165–174.
Vacher, L., Thivat, E., Poirier, C., Mouret-Reynier, M.A., Chollet, P., Devaud, H., . . . Chevrier, R. (2020). Improvement in adherence to capecitabine and lapatinib by way of a therapeutic education program. Supportive Care in Cancer, 28(7), 3313–3322. https://doi.org/10.1007/s00520-019-05144-x
Vrijens, B., De Geest, S., Hughes, D.A., Przemslaw, K., Demonceau, J., Ruppar, T., . . . Urquart, J. (2012). A new taxonomy for describing and defining adherence to medications. British Journal of Clinical Pharmacology, 73(5), 691–705. https://doi.org/10.1111/j.1365-2125.2012.04167.x
Wong, S.F., Bounthavong, M., Nguyen, C., Bechtoldt, K., & Hernandez, E. (2014). Implementation and preliminary outcomes of a comprehensive oral chemotherapy management clinic. American Journal of Health-System Pharmacy, 71(11), 960–965. https://doi.org/10.2146/ajhp130278
Zerillo, J.A., Goldenberg, B.A., Kotecha, R.R., Tewari, A.K., Jacobson, J.O., & Krzyzanowska, M.K. (2018). Interventions to improve oral chemotherapy safety and quality: A systematic review. JAMA Oncology, 4(1), 105–117.




