Ipilimumab-Based Therapy: Consensus Statement From the Faculty of the Melanoma Nursing Initiative on Managing Adverse Events With Ipilimumab Monotherapy and Combination Therapy With Nivolumab
Background: Ipilimumab (Yervoy®) therapy improves outcomes in patients with resected stage III melanoma, and ipilimumab alone or combined with nivolumab (Opdivo®) does so in those with unresectable or metastatic melanoma. These immunotherapies are associated with immune-related adverse events (irAEs). With prompt recognition and appropriate management, serious sequelae or unnecessary treatment discontinuation can be prevented.
Objectives: This article presents consensus statements to guide oncology nurses in the recognition and management of irAEs associated with ipilimumab and nivolumab.
Methods: Members of the Melanoma Nursing Initiative reviewed the current literature and clinical experience regarding nursing interventions related to irAEs associated with ipilimumab or ipilimumab and nivolumab therapy.
Findings: The care step pathways provided represent a proactive, evidence-based, and comprehensive plan to support optimal patient outcomes.
Jump to a section
Melanoma outcomes have been dramatically advanced by the development of immune checkpoint inhibitors (ICIs), which impede either cytotoxic T lymphocyte–associated antigen 4 (CTLA4) or programmed cell death protein 1 (PD-1) to enhance the patient’s immune system recognition and attack on cancer. Ipilimumab (Yervoy®) is a CTLA4 inhibitor that improved survival in phase 3 trials in unresectable stage III and stage IV melanoma (advanced melanoma) (Hodi et al., 2010; Robert et al., 2011). In 2011, the U.S. Food and Drug Administration (FDA) approved ipilimumab for advanced melanoma. Subsequently, ipilimumab was shown to prolong recurrence-free and overall survival as an adjuvant therapy in resected stage III melanoma (Eggermont et al., 2016) and received FDA approval for this indication in 2015. In addition, dual checkpoint blockade with ipilimumab, plus the PD-1 inhibitor nivolumab (Opdivo®), provides longer progression-free survival versus ipilimumab alone in advanced melanoma (Larkin et al., 2015) and was approved for this use in 2014.
Immune checkpoints serve as on- or off-regulators. In particular, CTLA4 and PD-1 act as off, or “brake,” mechanisms for the immune system. These pathways are exploited through interruptions to the brake system (i.e., taking the brakes off the immune system through ICIs). Although CTLA4 and PD-1 inhibitors work by sustaining (T-cell) immune responses, anti-CTLA4 tends to act earlier in the process than PD-1 (Boutros et al., 2016). These differences may also account for the divergent efficacy and toxicity profiles of CTLA4 and PD-1 inhibitors when used as monotherapy (Buchbinder & Desai, 2016). In the CheckMate 067 trial by Larkin et al. (2015), the group receiving ipilimumab and nivolumab therapy demonstrated higher response rates as well as increased progression-free survival versus ipilimumab monotherapy. However, the combination of ipilimumab and nivolumab was also associated with a higher proportion of severe toxicity: Fifty-five percent (n = 172) of patients in the combination therapy group experienced a treatment-related grade 3 or 4 adverse event, compared with 27% (n = 85) of those who received ipilimumab and 16% (n = 51) of those who received nivolumab, respectively (Larkin et al., 2015).
Members of the Melanoma Nursing Initiative (MNI) noted that the ipilimumab and nivolumab combination and high-dose ipilimumab adjuvant therapy are associated with the highest overall immune-related adverse event (irAE) rates and severity compared with other ICI regimens. The irAEs associated with these regimens also tend to have an earlier onset and faster progression versus other ICIs (see Figures 1, 2, and 3) (Boutros et al., 2016). Therefore, hypervigilance is warranted when monitoring patients who are receiving these regimens. 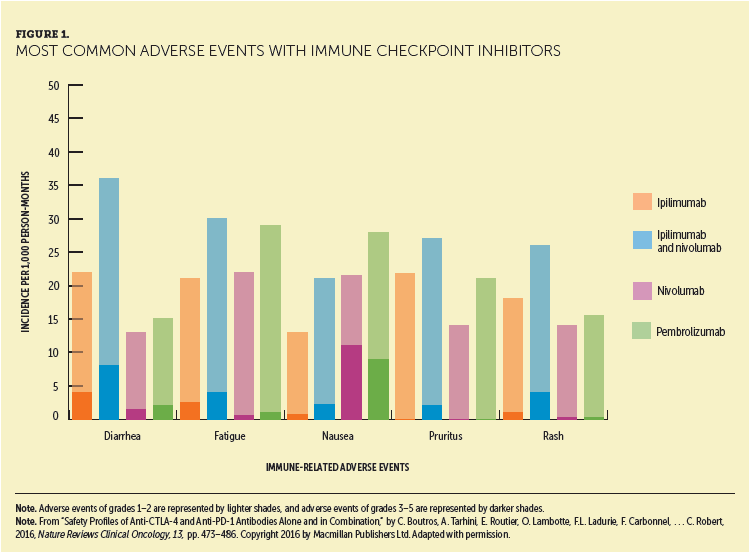
The MNI members convened to define supportive care challenges associated with ipilimumab-based therapy. Drawing from their clinical experience as well as review of the literature, they developed consensus recommendations for nursing care of this population. Recommendations were made in the following areas:
• Administration and dosing, with information on recommended drug holds and discontinuations
• irAE recognition and management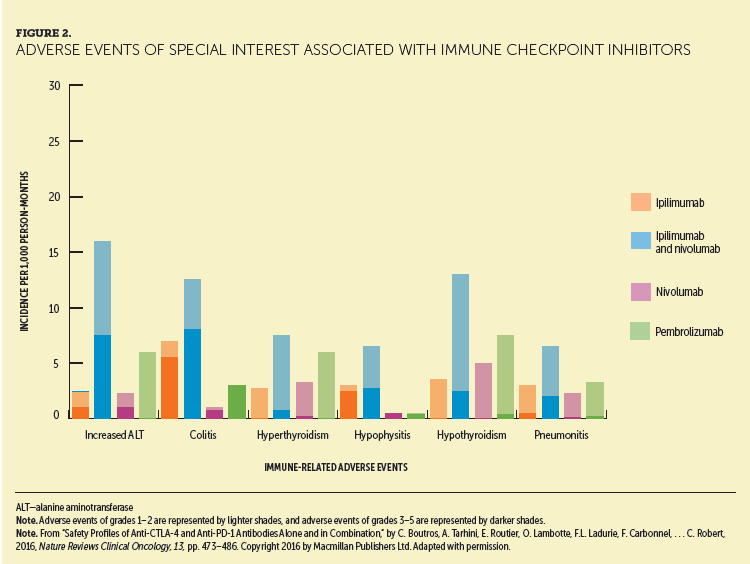
Care step pathways (CSPs) were developed for high-priority irAEs associated with ICIs, for which nursing assessment and care are key in promoting successful outcomes. This article focuses on irAE recognition and management principles specific to ipilimumab-based therapy. 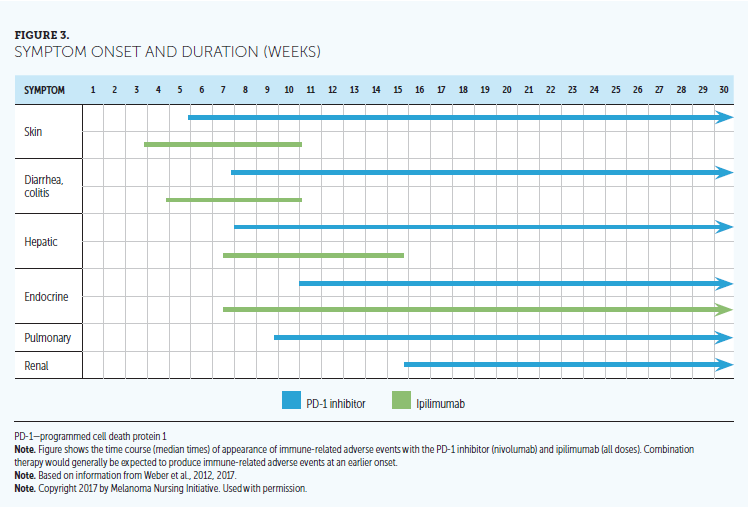
Drug Administration and Dosages
Information on ipilimumab and nivolumab dosing by indication is provided in Table 1 (Bristol-Myers Squibb, 2017a, 2017c). No premedications are given to prevent irAEs. Toxicity management may vary by the specific irAE, and more detail is discussed in the current article’s CSPs and tables. Of note, a much higher dose of ipilimumab (10 mg/kg versus 3 mg/kg) and a longer treatment duration are used as adjuvant treatment than are used as monotherapy or in combination therapy in the advanced melanoma setting. A dose-ranging study evaluating doses of 0.3 mg/kg, 3 mg/kg, and 10 mg/kg showed a clear dose-response effect on efficacy and toxicity for ipilimumab (Wolchok et al., 2010). Therefore, greater toxicity associated with the 10 mg/kg ipilimumab dose used for adjuvant therapy versus the 3 mg/kg dose used for metastatic disease may be expected (Eggermont et al., 2016). 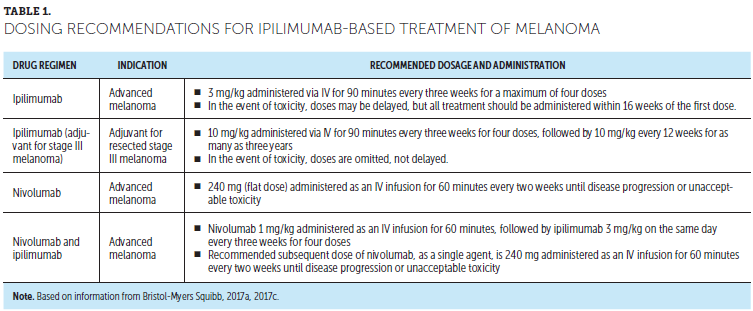
Nursing Interventions
Patient Advocacy in Therapy Selection
The best treatment approach in advanced melanoma remains debatable (i.e., whether to use a single agent versus a combination approach and how to sequence agents). Although nurses generally play a supportive role, they also serve as advocates for patient safety when treatment decisions are being made. In this capacity, nurses should assess for and highlight any concerns regarding barriers in physical resources (e.g., transportation, communication tools), as well as in personal support when patients undertake these effective but challenging regimens. Members of the MNI noted that PD-1 inhibitors are used more frequently than ipilimumab monotherapy in the metastatic setting, given the higher response rates and lower percentages of toxicity (Larkin et al., 2015; National Comprehensive Cancer Network [NCCN], 2016; Robert, Schacter, et al., 2015). Theefficacy–safety balance becomes a major discussion point when evaluating ipilimumab and nivolumab combination therapy versus PD-1 inhibitor monotherapy in this setting. In a similar way, the use of high-dose ipilimumab in the adjuvant setting is also informed by a benefit–risk discussion because of the potential for toxicities (NCCN, 2016).
General Education
Thorough, individualized, and comprehensible patient education is the critical first step of irAE management. Healthcare providers should emphasize to patients that any changes, even subtle variations in feeling, could be signs of an irAE and should be reported immediately. This is particularly important for patients who may be concerned about staying on therapy and who may feel the need to endure irAEs. Therefore, nurses need to form a strong partnership with patients and their caregivers to ensure that timely communication occurs. In the authors’ experience, patients sometimes encounter signs and symptoms that they think are attributable to the flu or a cold but that represent an irAE. Nurses should further emphasize that, although certain irAEs are more common than others and tend to occur at particular times following treatment initiation, ICIs can potentially affect any organ system or tissue and may occur at any time, including after treatment completion or discontinuation. Within this framework, the importance of obtaining baseline and follow-up testing to aid in irAE detection and diagnosis should be highlighted (Champiat et al., 2016).
Patients should also receive health maintenance information before therapy begins. Women of childbearing age should be counseled to avoid pregnancy because of the potential embryo-fetal toxicity associated with ICIs; effective birth control should be employed during therapy and for three months after the last dose (Bristol-Myers Squibb, 2017a; Friedman, Proverbs-Singh, & Postow, 2016). In addition, because ipilimumab is administered in the adjuvant setting for as many as three years, issues related to family planning may be highly relevant. Fertility studies have not been performed with ipilimumab (Bristol-Myers Squibb, 2017c). Given the long duration of therapy and the unknown impact on fertility in men and women, the oncology team should have open, candid discussions about family planning with patients of childbearing age and note any potential barriers or contraindications to future goals (Walter, Xu, Paller, Choi, & Woodruff, 2016). A possible recommendation may be referral to a fertility specialist to discuss potential sperm or egg banking as an option. Nurses continue to support the process by reinforcing conversations and addressing patient concerns.
Regarding other aspects of health maintenance, use of a sports team analogy may help the patient to understand the team-based approach employed and the central role of the oncologist. Patients may be counseled that their oncologist will become the quarterback of their care and, therefore, needs to have the picture of everything happening on the field. Patients should consult their oncology team before they receive elective surgery, dental procedures, or other medical interventions; start taking new medications or herbal supplements; or employ complementary modalities. The use of attenuated vaccines has been and continues to be evaluated. Live vaccines (e.g., measles, mumps, and rubella vaccine; varicella vaccine) have not been evaluated in this setting and are usually not advised (Bristol-Myers Squibb, 2017b; Merck, 2017). Nurses should counsel patients to discuss all immunizations with the oncology team to weigh the benefits and risks on an individual basis.
Oncology nurses should ensure that patients are aware of educational and financial resources for their therapies and how to access them (see Figure 4). In the experience of the MNI members, drug-specific wallet cards, which identify signs and symptoms that should be immediately reported to the treating oncologist or other members of the healthcare team, are particularly helpful. Patients should be encouraged to carry the card with them, even paperclipping their wallet card to their insurance card; by doing so, whenever they need to show their insurance card, they can share information about their immunotherapy regimen as well. Wallet cards not only serve as ready guides for notable signs and symptoms potentially reflective of an irAE but also provide relevant information to other healthcare providers, such as emergency department personnel, to improve patient care. 
Care Step Pathways
In general, irAEs observed with ipilimumab are similar to those associated with PD-1 inhibitor therapy, although they tend to occur more frequently and more severely. Ipilimumab acts early in the immunologic cascade, so many inflammatory AEs can occur. With the combination therapy, many irAEs occur even earlier and with greater severity. Optimal irAE management depends on early recognition, followed by appropriate strategies to minimize their impact (Boutros et al., 2016; Villadolid & Amin, 2015). Once identified, irAEs are typically managed symptomatically and/or with selective use of corticosteroids or other immunosuppressives, combined with withholding or discontinuation of ICIs, depending on the severity or persistence of the irAE (Boutros et al., 2016; Villadolid & Amin, 2015). Referral to an organ specialist is often warranted, particularly for more serious or severe irAEs or for those that the healthcare provider is not experienced in managing, such as endocrinopathies. With prompt recognition and appropriate treatment, some irAEs are reversible; endocrinopathies are an exception and are generally managed with lifelong hormone replacement therapy (HRT). With respect to corticosteroid use, some patients may be concerned that because corticosteroids are immunosuppressive, they may interfere with ICI efficacy. Nurses should assure patients that studies indicate that ICI efficacy is not adversely affected by corticosteroids or treatment of irAEs (Horvat et al., 2015; Weber et al., 2015).
Each CSP represents a notable irAE associated with ICIs and incorporates essential components of the nursing assessment specific to that irAE. Look, listen, and recognize categories within the nursing assessment section direct the nurse to a specific set of symptom-related queries to ask the patient and/or caregiver and highlight additional information to be considered and reviewed as part of the nursing assessment. Data obtained from this assessment will guide appropriate management strategies. Wherever possible, grading for the specific AE is provided within the pathway based on the National Cancer Institute’s (2010) Common Terminology Criteria for Adverse Events (CTCAE). However, for irAEs for which management is largely directed based on laboratory data (such as thyroid disorders and diabetes), the authors used standard cutpoint criteria rather than the CTCAE criteria.
Each CSP describes overall management strategies as well as nursing-specific interventions. Where applicable, interventions for at-risk groups along with strategies specific to each AE grade are listed, including dose holds and discontinuations. Patient counseling, recommendations for additional care, and referral to specialty or ancillary care providers are included in the management section, as appropriate.
Some general principles inform management strategies across all the CSPs. Corticosteroid dosages are detailed in the CSPs, and these high dosages are often split into two daily doses. Corticosteroids should be gradually tapered (usually for at least a month) to avoid rebound symptoms. Methylprednisolone dosepaks are used by some providers, but are often not sufficient to reduce higher grade inflammatory reactions resulting from the ICIs. Gastric prophylaxis with a proton pump inhibitor or H2 blocker should be considered when initiating high-dose corticosteroids. Patients should be educated to take their steroid dose with food. In addition, antimicrobial prophylaxis should be considered for patients prescribed high-dose steroids, particularly for those patients requiring extended therapy (greater than 20 mg per day for more than one month) (Limper et al., 2011).
Common Immune-Related Adverse Events
Skin Toxicities
Maculopapular rash and pruritus (without rash) are the most common irAEs associated with ICI treatment and are usually the first to appear (Dadu, Zobniw, & Diab, 2016; Friedman et al., 2016). Pruritic rash is more common in ipilimumab-based regimens. Rash with or without pruritus is usually mild to moderate and can be effectively managed with prompt treatment (Boutros et al., 2016; Dadu et al., 2016). Appendix A provides a detailed CSP for ICI-emergent rash or pruritus.
[[{"type":"media","view_mode":"media_original","fid":"34226","field_deltas":{"1":{}},"link_text":null,"fields":{},"attributes":{"height":"940","width":"752","class":"media-image media-element file-media-original","data-delta":"1"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34231","field_deltas":{"2":{}},"link_text":null,"fields":{},"attributes":{"height":"938","width":"764","class":"media-image media-element file-media-original","data-delta":"2"}}]]
Nurses must educate patients about the cutaneous irAEs, implement self-care strategies in at-risk individuals, and minimize the severity of irAEs that do develop. Self-care strategies, such as gentle skin care and use of moisturizers and sun-protective techniques, are important early interventions for rash and pruritus, particularly in at-risk patients (those with preexisting conditions, such as dermatitis or xerosis). If patients develop skin-related toxicities, the nurse should grade the toxicity, provide appropriate supportive care interventions, and work as part of the team for management. The role of treatment holds and the use of topical and oral steroids are discussed in the CSP. Rarely, skin toxicities can progress and become severe or life-threatening; consequently, ongoing vigilance is necessary to promptly recognize red flags, such as extensive or rapidly progressive rash, oral involvement, or other indicators of Stevens-Johnson syndrome or toxic epidermal necrolysis, which represent medical emergencies that typically require hospitalization.
Gastrointestinal Toxicities
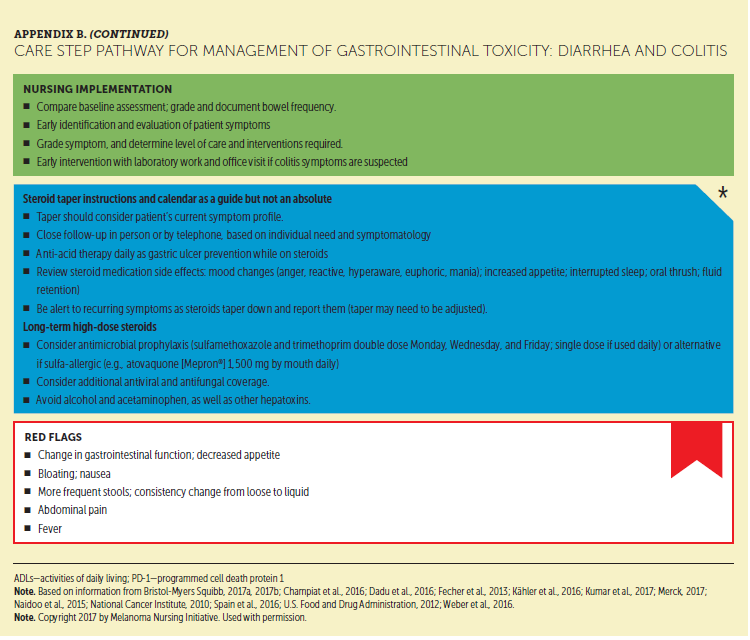
Gastrointestinal toxicities are the second most common irAEs observed with ICI therapy, and they range from mild diarrhea to severe colitis and intestinal perforation, which can lead to death (Cramer & Bresalier, 2017; Dadu et al., 2016; Gupta, De Felice, Loftus, & Khanna, 2015). Diarrhea without symptoms of colitis is the most common gastrointestinal presentation with ICIs (Cramer & Bresalier, 2017; Dadu et al., 2016; Gupta et al., 2015). Abdominal pain, together with mucus and/or blood in the stool, is suggestive of colitis rather than simple diarrhea (Gupta et al., 2015). In an analysis of ICI irAEs, ipilimumab-induced colitis was the most common cause of fatal irAEs across clinical trials (De Velasco et al., 2017). The colitis associated with high-dose (10 mg/kg) ipilimumab and the combination regimen is generally more severe and has a quicker onset than that associated with PD-1 inhibitor monotherapy. As a result, early reporting from patients is critical, and diligence is required in the clinic to recognize and manage this irAE early.
Appendix B provides a detailed CSP for ICI-emergent gastrointestinal toxicities. Nurses play a vital role in the early identification and grading of these toxicities by comparing baseline measures of bowel movements to current status. They may arrange a laboratory workup and/or office visit, when appropriate. Non-ICI–related causes of diarrhea need to be ruled out to ensure proper treatment (Dadu et al., 2016; Gupta et al., 2015). Because colitis can progress to severe or life-threatening forms, hospitalization is often required with gastrointestinal consultation, and surgical consultation is needed if peritoneal signs are noted (e.g., poor appetite, nausea, abdominal pain or tenderness aggravated by movement, distension) and perforation is suspected. For diarrhea that is moderate to severe, corticosteroids are key to managing symptoms. However, if a patient is not responsive to corticosteroid therapy, the addition of infliximab at 5 mg/kg may be considered, followed by slow taper until diarrhea symptoms resolve (Dadu et al., 2016).
[[{"type":"media","view_mode":"media_original","fid":"34236","field_deltas":{"3":{}},"link_text":null,"fields":{},"attributes":{"height":"771","width":"751","class":"media-image media-element file-media-original","data-delta":"3"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34241","field_deltas":{"4":{}},"link_text":null,"fields":{},"attributes":{"height":"936","width":"753","class":"media-image media-element file-media-original","data-delta":"4"}}]]
ICIs may also adversely affect the proximal portion of the gastrointestinal system, causing inflammation of the oral mucosa and leading to mucositis and xerosis. Appendix C provides a detailed CSP for ICI-emergent mucositis and xerostomia. These irAEs may be easily overlooked but can negatively affect patients’ quality of life. Nurses need to recognize the immune-related causation, which leads to a different management strategy versus that used for mucositis associated with chemotherapy.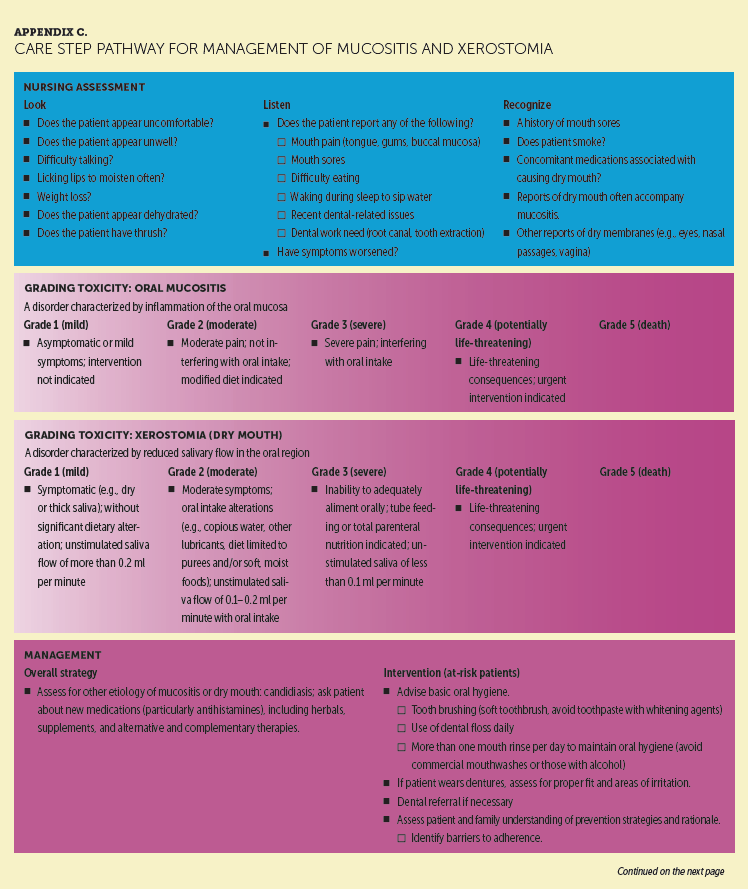

Hepatotoxicity
ICIs commonly produce hepatitis, which typically manifests as elevated alanine aminotransferase or aspartateaminotransferase levels (Dadu et al., 2016; Friedman et al., 2016). Liver transaminase elevations are observed less frequently with ICIs than skin or gastrointestinal toxicities, but relatively high rates have been reported with the ipilimumab and nivolumab combination or high-dose ipilimumab therapy (Eggermont et al., 2015; Larkin et al., 2015). Asymptomatic, improperly managed hepatic irAEs can lead to mortality (Dadu et al., 2016). Appendix D provides a detailed CSP for ICI-emergent hepatotoxicity. The authors recommend that nurses closely monitor liver function tests (LFTs) in patients receiving combination therapy. LFTs are generally warranted prior to each immunotherapy dose. Transaminase grading may guide the frequency of LFT checks. Given the vital importance and complex functionality of the liver, distinguishing between true organ dysfunction versus immune-mediated issues can be difficult (De Velasco et al., 2017). Consequently, consultation with a hepatologist or gastroenterologist should be considered. A liver biopsy may be necessary to differentiate autoimmune toxicity from other causes of liver injury. Management strategies for moderate to life-threatening hepatotoxicity are high-dose corticosteroids at 1–2 mg/kg. If LFTs remain elevated and refractory to steroids, additional immunosuppression may be considered with the use of mycophenolate mofetil at 500–1,000 mg by mouth every 12 hours until LFTs are stable or declining. Medications should be tapered slowly until resolution of hepatitis (Dadu et al., 2016). Ensuring adherence to the mycophenolate regimen is essential in providing optimal outcomes for these patients.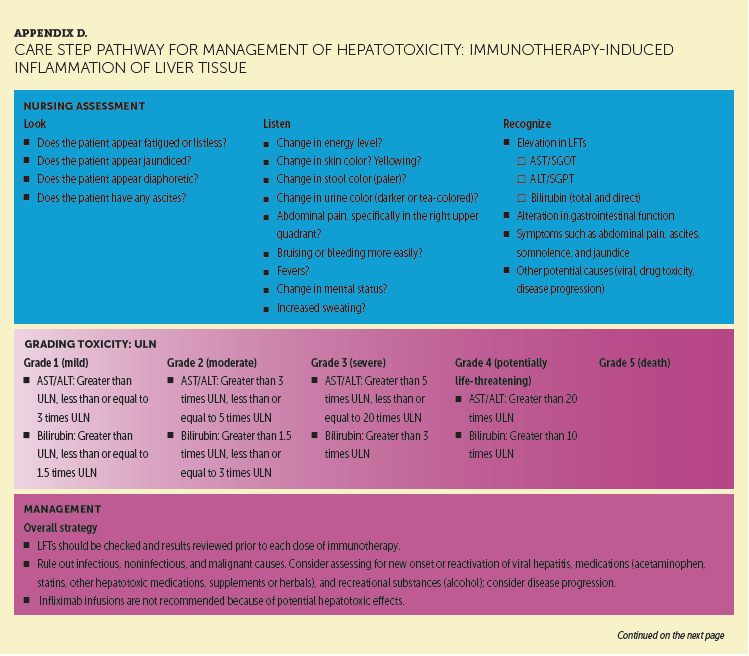
[[{"type":"media","view_mode":"media_original","fid":"34246","field_deltas":{"5":{}},"link_text":null,"fields":{},"attributes":{"height":"827","width":"750","class":"media-image media-element file-media-original","data-delta":"5"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34251","field_deltas":{"6":{}},"link_text":null,"fields":{},"attributes":{"height":"545","width":"750","class":"media-image media-element file-media-original","data-delta":"6"}}]]
Rarer But Serious Immune-Related Adverse Events
Endocrinopathies
Endocrinopathies associated with ICIs occur because the overactive immune system damages endocrine organs, mimicking the autoimmune process. Endocrinopathies are less common than skin or gastrointestinal irAEs, but they can have serious consequences if not recognized and properly treated (Byun, Wolchok, Rosenberg, & Girotra, 2017). They include hypophysitis, thyroiditis, and type 1 or insulin-dependent diabetes mellitus (Dadu et al., 2016; Friedman et al., 2016). A range of endocrine organs can be affected, including the pituitary, thyroid, pancreas, and adrenal glands. Although relatively rare, these endocrinopathies can lead to serious sequelae. Hypophysitis may lead to various hormone deficiencies, including central hypothyroidism, hypogonadism, and/or adrenal insufficiency. Similarly, thyroiditis can lead to hyperthyroidism, often followed by hypothyroidism. Nurses should be aware that endocrinopathies often present with vague symptoms (e.g., fatigue, headache, depression) that require further evaluation. They should discuss with patients, at the onset, that ICIs typically produce endocrine conditions requiring lifelong HRT. Patients are usually more willing to accept ICI therapy when they understand they can generally lead normal lives with HRT.
[[{"type":"media","view_mode":"media_original","fid":"34256","field_deltas":{"7":{}},"link_text":null,"fields":{},"attributes":{"height":"700","width":"744","class":"media-image media-element file-media-original","data-delta":"7"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34261","field_deltas":{"8":{}},"link_text":null,"fields":{},"attributes":{"height":"639","width":"752","class":"media-image media-element file-media-original","data-delta":"8"}}]]
Appendixes E and F provide detailed CSPs for ICI-emergent hypophysitis and thyroiditis, respectively. Particularly when addressing hypophysitis, the nurse should help facilitate collaborative management with an experienced endocrinologist to properly address the challenging diagnostic requirements for this condition. Such collaboration represents the best approach to care, although some oncology practices will become more independent in their management of these irAEs over time.
[[{"type":"media","view_mode":"media_original","fid":"34266","field_deltas":{"9":{}},"link_text":null,"fields":{},"attributes":{"height":"484","width":"749","class":"media-image media-element file-media-original","data-delta":"9"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34271","field_deltas":{"10":{}},"link_text":null,"fields":{},"attributes":{"height":"861","width":"752","class":"media-image media-element file-media-original","data-delta":"10"}}]]
Type 1 diabetes mellitus is a much rarer endocrinopathy linked with ICI therapy (Dadu et al., 2016; Friedman et al., 2016), but it can be disconcerting for patients, given the potential serious consequences. The condition is caused by autoimmune destruction of pancreatic beta cells (Chae et al., 2017) and is a permanent condition managed with insulin therapy. Nurses play an essential role in educating patients regarding this potential irAE, monitoring laboratory tests and patient symptoms, and ensuring prompt and comprehensive management if the patient develops type 1 diabetes mellitus. Appendix G provides a detailed CSP for ICI-emergent type 1 diabetes mellitus. 
Pneumonitis
Pneumonitis is a rare but potentially fatal irAE. Like endocrinopathies, pneumonitis tends to occur later than most other ICI-associated irAEs; however, like all irAEs, pneumonitis may occur and should be anticipated at any time. Highest rates are observed with ipilimumab and nivolumab combination therapy (Dadu et al., 2016; Friedman et al., 2016). This condition is frequently diagnosed based on imaging, such as follow-up computed tomography tests, because it is often asymptomatic. Nurses should consider a referral to a pulmonary specialist for patients presenting with nonspecific pulmonary symptoms, such as upper respiratory tract infection, new or persistent cough, or dyspnea. In early phases, pneumonitis may present as dyspnea on exertion, which the nurse may evaluate through pulse oxygen monitoring in the clinic. Patients who develop abnormalities in such testing can be considered for further radiologic testing. Appendix H provides a detailed CSP for ICI-emergent pneumonitis. 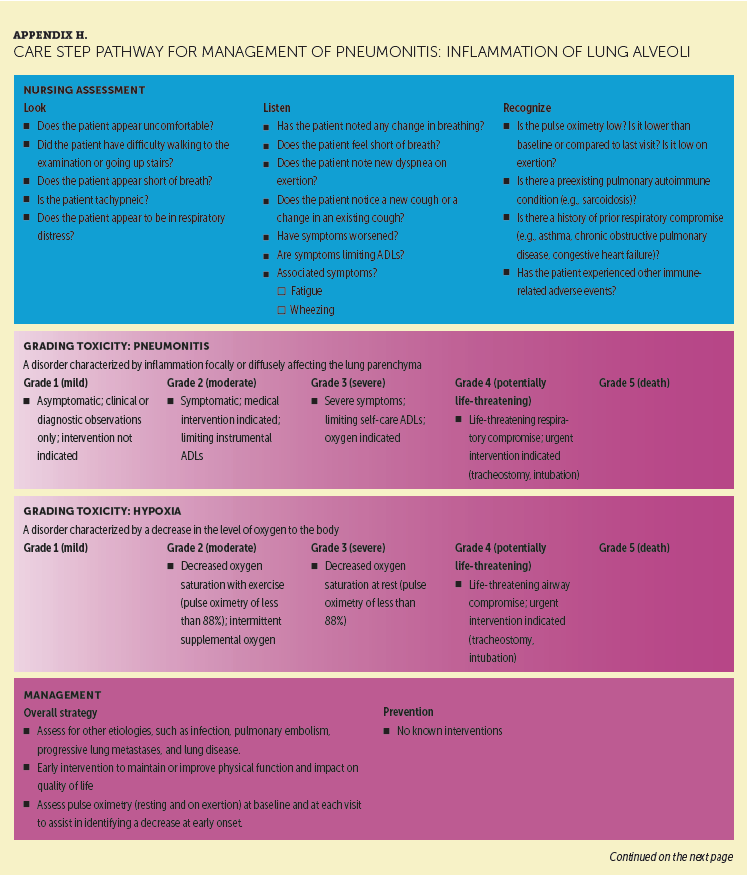

Easily Overlooked and Other Immune-Related Adverse Events
ICIs can potentially affect any body organ or tissue. Other irAEs have been reported with ipilimumab and/or nivolumab, highlighting the wide range of affected tissues (Bristol-Myers Squibb, 2017a, 2017c). Of particular importance are those affecting the musculoskeletal, nervous, and renal systems. Appendix I provides a detailed CSP for ICI-emergent arthralgias and arthritis, which were reported in more than 10% of patients receiving ipilimumab and nivolumab combination therapy (Cappelli, Shah, & Bingham, 2017; Larkin et al., 2015). If not anticipated, these irAEs are easily overlooked or not associated with ICIs. Permanent joint damage may occur without treatment, and referral to a rheumatologist is sometimes warranted (Cappelli, Naidoo, Bingham, & Shah, 2017). Arthralgias or arthritis often require more prolonged anti-inflammatory treatment than other irAEs. However, the authors reported some cases in which low-dose prednisone can have a major effect on quality of life, particularly in older adults. 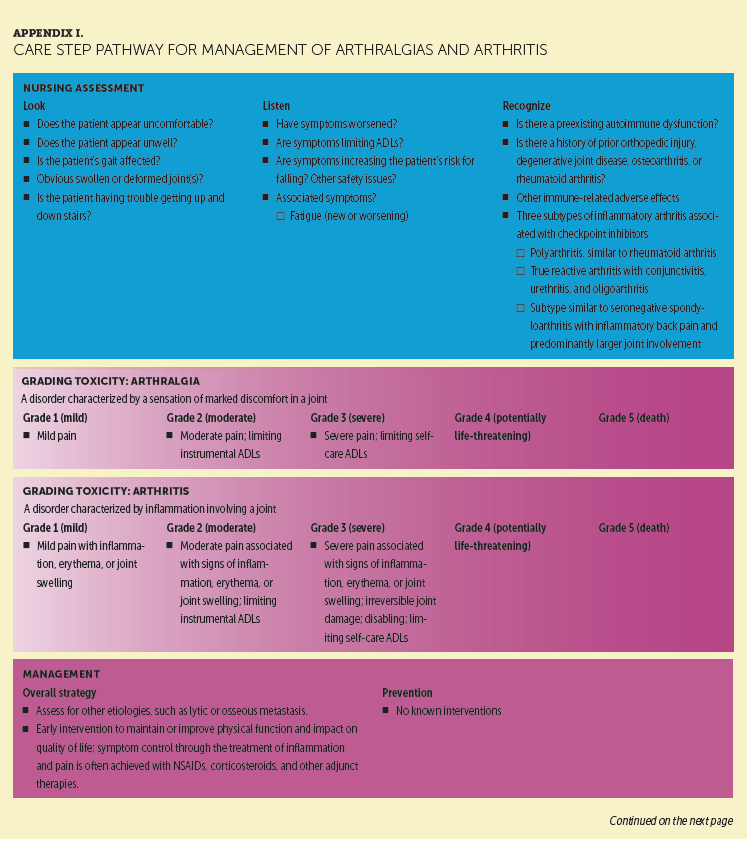
[[{"type":"media","view_mode":"media_original","fid":"34276","field_deltas":{"11":{}},"link_text":null,"fields":{},"attributes":{"height":"920","width":"752","class":"media-image media-element file-media-original","data-delta":"11"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34281","field_deltas":{"12":{}},"link_text":null,"fields":{},"attributes":{"height":"347","width":"747","class":"media-image media-element file-media-original","data-delta":"12"}}]]
Appendix J provides a detailed CSP for ICI-emergent neuropathy, specifically motor or sensory nerve impairment or damage. ICIs can cause rare but serious and life-threatening neurologic irAEs, including rapidly progressing encephalitis. For example, encephalopathy can be initially mistaken for an endocrinopathy before rapidly progressing from headache to confusion to coma. Prompt referral to a neurologist for further evaluation is warranted. Many of the symptoms for the irAEs overlap (e.g., headache), and patients often provide only vague descriptions. Therefore, accurate diagnosis can be delayed, leading to poorer outcomes. Being aware of the range of toxicities that are possible is important, and immune dysfunction should be considered for every complaint or symptom presentation.
[[{"type":"media","view_mode":"media_original","fid":"34286","field_deltas":{"13":{}},"link_text":null,"fields":{},"attributes":{"height":"865","width":"755","class":"media-image media-element file-media-original","data-delta":"13"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34291","field_deltas":{"14":{}},"link_text":null,"fields":{},"attributes":{"height":"428","width":"750","class":"media-image media-element file-media-original","data-delta":"14"}}]]
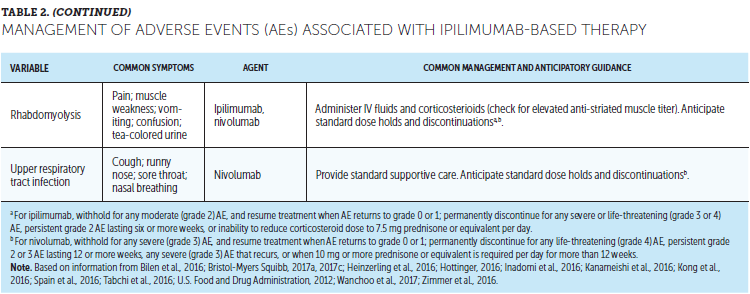
Appendix K provides a detailed CSP for ICI-emergent nephritis. Nurses should make sure that patients are monitored for elevated serum creatinine before and periodically during ICI therapy and referred for appropriate treatment when needed. Table 2 lists other AEs reported with ipilimumab and/or nivolumab treatment that may be directly related to the immune effects of these agents as well as recommended management strategies.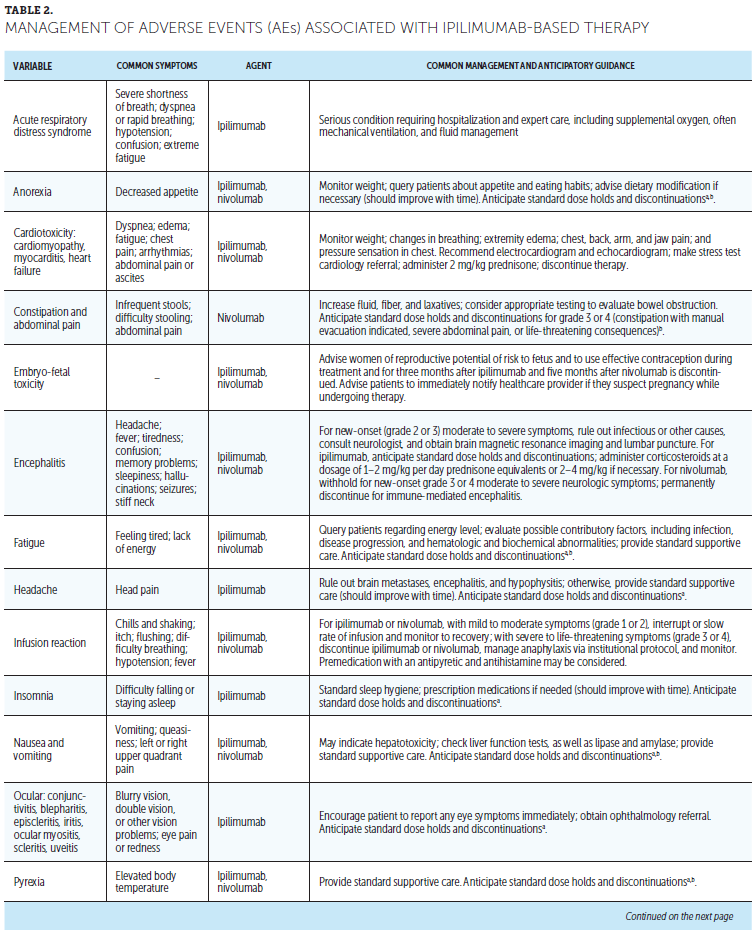
Immunotherapy-induced cardiotoxicity, albeit rare, has been noted to have fatal outcomes in a small number of patients who developed myocarditis or cardiomyopathy (Heinzerling et al., 2016; Johnson et al., 2016). This area is gaining increasing awareness and attention as an irAE. Cardiotoxicity should be considered in patients reporting vague or atypical symptoms, particularly those that are cardiorespiratory in nature (e.g., shortness of breath, weakness, edema). Patients suspected of experiencing a cardiotoxicity should be evaluated immediately (Heinzerling et al., 2016; Johnson et al., 2016).
[[{"type":"media","view_mode":"media_original","fid":"34296","field_deltas":{"15":{}},"link_text":null,"fields":{},"attributes":{"height":"936","width":"754","class":"media-image media-element file-media-original","data-delta":"15"}}]]
[[{"type":"media","view_mode":"media_original","fid":"34301","field_deltas":{"16":{}},"link_text":null,"fields":{},"attributes":{"height":"934","width":"760","class":"media-image media-element file-media-original","data-delta":"16"}}]]
Conclusion
Ipilimumab-based therapy (high-dose adjuvant monotherapy, ipilimumab monotherapy, or ipilimumab and nivolumab combination therapy for advanced disease) improves survival outcomes in patients with melanoma. However, ipilimumab monotherapy and combination therapy produce irAEs unlike those of traditional or newer targeted treatments and at higher rates and severity than with ICI monotherapy. Optimal clinical outcomes in these patient populations depend, in part, on early detection and management of ipilimumab-related irAEs. 
Oncology nurses can have a positive impact on outcomes by educating patients about the importance of immediately reporting changes in health status, providing patient support throughout the treatment process, assessing patient adherence and understanding of the dosing regimen, regularly evaluating patient status and potential symptoms, and serving as a liaison among the patient, the medical team, and consulting specialists. By familiarizing themselves with the CSPs presented in this article, nurses will be well prepared to provide the comprehensive patient assistance and support needed to maximize treatment outcomes.
The authors gratefully acknowledge Jill Maria Weberding, MPH, BSN, RN, OCN®, for reviewing the manuscript from the community oncology nursing perspective.
About the Author(s)
Kathleen M. Madden, RN, MSN, FNP-BC, AOCNP®, APHN, is a family nurse practitioner in the melanoma/medical oncology group of the Laura and Isaac Perlmutter Cancer Center at the New York University Langone Medical Center in New York, NY; and Brianna Hoffner, RN, MSN, APN-BC, AOCNP®, is a lead advanced practice provider for medical oncology at the University of Colorado Cancer Center in Aurora. The authors take full responsibility for this content. This supplement was funded by the AIM at Melanoma Foundation, with support via unrestricted grants from Amgen, Array Biopharma, Bristol-Myers Squibb, Incyte Corporation, Merck and Co., and Novartis Pharmaceuticals. Writing and editorial support was provided by Michael L. Coco, PhD, of Coco Communications, Inc., Lisa A. Tushla, PhD, H(ASCP), of Terranova Medica, and Marjorie Joyce, BA. Madden has previously consulted for and has received payment for services on speakers bureaus from Bristol-Myers Squibb and Merck and Co. Hoffner has previously served on speakers bureaus for Bristol-Myers Squibb, Genentech, Merck and Co., and Novartis Pharmaceuticals. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. Madden can be reached at kathleen.madden@nyumc.org, with copy to CJONEditor@ons.org. (Submitted April 2017. Accepted June 6, 2017.)
References
Bilen, M.A., Subudhi, S.K., Gao, J., Tannir, N.M., Tu, S.M., & Sharma, P. (2016). Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. Journal for Immunotherapy of Cancer, 4, 36. doi:10.1186/s40425-016-0139-8
Boutros, C., Tarhini, A., Routier, E., Lambotte, O., Ladurie, F.L., Carbonnel, F., . . . Robert, C. (2016). Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nature Reviews Clinical Oncology, 13, 473–486. doi:10.1038/nrclinonc.2016.58
Bristol-Myers Squibb. (2017a). Opdivo® (nivolumab) [Package insert]. Retrieved from http://www.opdivoyervoyhcp.com
Bristol-Myers Squibb. (2017b, February 21). Use of vaccines in patients receiving nivolumab [Standard response letter to Lisa Tushla, Terranova Medica, LLC].
Bristol-Myers Squibb. (2017c). Yervoy® (ipilimumab) [Package insert]. Retrieved from http://packageinserts.bms.com/pi/pi_yervoy.pdf
Buchbinder, E.I., & Desai, A. (2016). CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. American Journal of Clinical Oncology, 39, 98–106. doi:10.1097/COC.0000000000000239
Byun, D.J., Wolchok, J.D., Rosenberg, L.M., & Girotra, M. (2017). Cancer immunotherapy—Immune checkpoint blockade and associated endocrinopathies. Nature Reviews Endocrinology, 13, 195–207. doi:10.1038/nrendo.2016.205
Cappelli, L.C., Naidoo, J., Bingham, C.O., III, & Shah, A.A. (2017). Inflammatory arthritis due to immune checkpoint inhibitors: Challenges in diagnosis and treatment. Immunotherapy, 9, 5–8. doi:10.2217/imt-2016-0117
Cappelli, L.C., Shah, A.A., & Bingham, C.O., III. (2017). Immune-related adverse effects of cancer immunotherapy—Implications for rheumatology. Rheumatic Diseases Clinics of North America, 43, 65–78. doi:10.1016/j.rdc.2016.09.007
Chae, Y.K., Chiec, L., Mohindra, N., Gentzler, R., Patel, J., & Giles, F. (2017). A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunology, Immunotherapy, 66, 25–32. doi:10.1007/s00262-016-1913-7
Champiat, S., Lambotte, O., Barreau, E., Belkhir, R., Berdelou, A., Carbonnel, F., . . . Marabelle, A. (2016). Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Annals of Oncology, 27, 559–574. doi:10.1093/annonc/mdv623
Cramer, P., & Bresalier, R.S. (2017). Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Current Gastroenterology Reports, 19, 3. doi:10.1007/s11894-017-0540-6
Dadu, R., Zobniw, C., & Diab, A. (2016). Managing adverse events with immune checkpoint agents. Cancer Journal, 22, 121–129. doi:10.1097/PPO.0000000000000186
De Velasco, G., Je, Y., Bossé, D., Awad, M.M., Ott, P.A., Moreira, R.B., . . . Choueiri, T.K. (2017). Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L inhibitors in cancer patients. Cancer Immunology Research, 5, 312–318. doi:10.1158/2326-6066.CIR-16-0237
Eggermont, A.M., Chiarion-Sileni, V., Grob, J.J., Dummer, R., Wolchok, J.D., Schmidt, H., . . . Testori, A. (2015). Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncology, 16, 522–530. doi:10.1016/S1470-2045(15)70122-1
Eggermont, A.M., Chiarion-Sileni, V., Grob, J.-J., Dummer, R., Wolchok, J.D., Schmidt, H., . . . Testori, A. (2016). Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. New England Journal of Medicine, 375, 1845–1855. doi:10.1056/NEJMoa1611299
Friedman, C.F., Proverbs-Singh, T.A., & Postow, M.A. (2016). Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncology, 2, 1346–1353. doi:10.1001/jamaoncol.2016.1051
Gupta, A., De Felice, K.M., Loftus, E.V., Jr., & Khanna, S. (2015). Systematic review: Colitis associated with anti-CTLA-4 therapy. Alimentary Pharmacology and Therapeutics, 42, 406–417. doi:10.1111/apt.13281
Heinzerling, L., Ott, P.A., Hodi, F.S., Husain, A.N., Tajmir-Riahi, A., Tawbi, H., . . . Luke, J.J. (2016). Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. Journal for ImmunoTherapy of Cancer, 4, 50. doi:10.1186/s40425-016-0152-y
Hodi, F.S., O’Day, S.J., McDermott, D.F., Weber, R.W., Sosman, J.A., Haanen, J.B., . . . Urba, W.J. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine, 363, 711–723. doi:10.1056/NEJMoa1003466
Horvat, T.Z., Adel, N.G., Dang, T.-O., Momtaz, P., Postow, M.A., Callahan, M.K., . . . Chapman, P.B. (2015). Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. Journal of Clinical Oncology, 33, 3193–3198. doi:10.1200/JCO.2015.60.8448
Hottinger, A.F. (2016). Neurologic complications of immune checkpoint inhibitors. Current Opinion in Neurology, 29, 806–812. doi:10.1097/WCO.0000000000000391
Inadomi, K., Kumagai, H., Arita, S., Tsuruta, N., Takayoshi, K., Mishima, K., . . . Baba, E. (2016). Bi-cytopenia possibly induced by anti-PD-1 antibody for primary malignant melanoma of the esophagus: A case report. Medicine, 95, e4283. doi:10.1097/MD.0000000000004283
Johnson, D.B., Balko, J.M., Compton, M.L., Chalkias, S., Gorham, J., Xu, Y., . . . Moslehi, J.J. (2016). Fulminant myocarditis with combination immune checkpoint blockade. New England Journal of Medicine, 375, 1749–1755. doi:10.1056/NEJMoa1609214
Kanameishi, S., Otsuka, A., Nonomura, Y., Fujisawa, A., Endo, Y., & Kabashima, K. (2016). Idiopathic thrombocytopenic purpura induced by nivolumab in a metastatic melanoma patient with elevated PD-1 expression on B cells. Annals of Oncology, 27, 546–547. doi:10.1093/annonc/mdv580
Kong, B.Y., Micklethwaite, K.P., Swaminathan, S., Kefford, R.F., & Carlino, M.S. (2016). Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Research, 26, 202–204. doi:10.1097/CMR.0000000000000232
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J.J., Cowey, C.L., Lao, C.D., . . . Wolchok, J.D. (2015). Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New England Journal of Medicine, 373, 23–34. doi:10.1056/NEJMoa1504030
Limper, A.H., Knox, K.S., Sarosi, G.A., Ampel, N.M., Bennett, J.E., Catanzaro, A., . . . Stevens, D.A. (2011). An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. American Journal of Respiratory and Critical Care Medicine, 183, 96–128. doi:10.1164/rccm.2008-740ST
Merck. (2017, February 17). Use with live attenuated vaccines [Standard response letter to Kathleen Marie Madden, NP].
National Cancer Institute. (2010). Common Terminology Criteria for Adverse Events [v.4.03]. Retrieved from https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference…
National Comprehensive Cancer Network. (2016). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Melanoma [v.1.2017]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf
Robert, C., Schachter, J., Long, G.V., Arance, A., Grob, J.J., Mortier, L., . . . Ribas, A. (2015). Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine, 372, 2521–2532. doi:10.1056/NEJMoa1503093
Robert, C., Thomas, L., Bondarenko, I., O’Day, S., Weber, J., Garbe, C., . . . Wolchok, J.D. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England Journal of Medicine, 364, 2517–2526. doi:10.1056/NEJMoa1104621
Spain, L., Diem, S., & Larkin, J. (2016). Management of toxicities of immune checkpoint inhibitors. Cancer Treatment Reviews, 44, 51–60. doi:10.1016/j.ctrv.2016.02.001
Tabchi, S., Weng, X., & Blais, N. (2016). Severe agranulocytosis in a patient with metastatic non-small-cell lung cancer treated with nivolumab. Lung Cancer, 99, 123–126. doi:10.1016/j.lungcan.2016.06.026
U.S. Food and Drug Administration. (2012). Risk evaluation and mitigation strategy (REMS). Retrieved from https://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInfo…
Villadolid, J., & Amin, A. (2015). Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Translational Lung Cancer Research, 4, 560–575. doi:10.3978/j.issn.2218-6751.2015.06.06
Walter, J.R., Xu, S., Paller, A.S., Choi, J.N., & Woodruff, T.K. (2016). Oncofertility considerations in adolescents and young adults given a diagnosis of melanoma: Fertility risk of Food and Drug Administration–approved systemic therapies. Journal of the American Academy of Dermatology, 75, 528–534. doi:10.1016/j.jaad.2016.04.031
Wanchoo, R., Karam, S., Uppal, N.N., Barta, V.S., Deray, G., Devoe, C., . . . Jhaveri, K.D. (2017). Adverse renal effects of immune checkpoint inhibitors: A narrative review. American Journal of Nephrology, 45, 160–169. doi:10.1159/000455014
Weber, J.S., D’Angelo, S.P., Minor, D., Hodi, F.S., Gutzmer, R., Neyns, B., . . . Larkin, J. (2015). Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncology, 16, 375–384. doi:10.1016/S1470-2045(15)70076-8
Weber, J.S., Hodi, F.S., Wolchok, J.D., Topalian, S.L., Schadendorf, D., Larkin, J., . . . Robert, C. (2017). Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. Journal of Clinical Oncology, 35, 785–792. doi:10.1200/JCO.2015.66.1389
Weber, J.S., Kähler, K.C., & Hauschild, A. (2012). Management of immune-related adverse events and kinetics of response with ipilimumab. Journal of Clinical Oncology, 30, 2691–2697. doi:10.1200/JCO.2012.41.6750
Wolchok, J.D., Neyns, B., Linette, G., Negrier, S., Lutzky, J., Thomas, L., . . . Lebbé, C. (2010). Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncology, 11, 155–164. doi:10.1016/S1470-2045(09)70334-1
Zimmer, L., Goldinger, S.M., Hofmann, L., Loquai, C., Ugurel, S., Thomas, I., . . . Heinzerling, L.M. (2016). Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. European Journal of Cancer, 60, 210–225. doi:10.1016/j.ejca.2016.02.024
References for Appendixes A-K
Bristol-Myers Squibb. (2017a). Opdivo® (nivolumab) [Package insert]. Retrieved from http://www.opdivoyervoyhcp.com
Bristol-Myers Squibb. (2017b). Yervoy® (ipilimumab) [Package insert]. Retrieved from http://packageinserts.bms.com/pi/pi_yervoy.pdf
Byun, D.J., Wolchok, J.D., Rosenberg, L.M., & Girotra, M. (2017). Cancer immunotherapy—Immune checkpoint blockade and associated endocrinopathies. Nature Reviews Endocrinology, 13, 195–207.
Cappelli, L.C., Naidoo, J., Bingham, C.O., III, & Shah, A.A. (2017). Inflammatory arthritis due to immune checkpoint inhibitors: Challenges in diagnosis and treatment. Immunotherapy, 9, 5–8.
Cappelli, L.C., Shah, A.A., & Bingham, C.O., III. (2017). Immune-related adverse effects of cancer immunotherapy—Implications for rheumatology. Rheumatic Diseases Clinics of North America, 43, 65–78.
Champiat, S., Lambotte, O., Barreau, E., Belkhir, R., Berdelou, A., Carbonnel, F., . . . Marabelle, A. (2016). Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Annals of Oncology, 27, 559–574.
Dadu, R., Zobniw, C., & Diab, A. (2016). Managing adverse events with immune checkpoint agents. Cancer Journal, 22, 121–129.
Durham, C.O., Fowler, T., Donato, A., Smith, W., & Jensen, E. (2015). Pain management in patients with rheumatoid arthritis. Nurse Practitioner, 40(5), 38–45. doi:10.1097/01.NPR.0000463784.36883.23
Fecher, L.A., Agarwala, S.S., Hodi, F.S., & Weber, J.S. (2013). Ipilimumab and its toxicities: A multidisciplinary approach. Oncologist, 18, 733–743. doi:10.1634/theoncologist.2012-0483
Friedman, C.F., Proverbs-Singh, T.A., & Postow, M.A. (2016). Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncology, 2, 1346–1353. doi:10.1001/jamaoncol.2016.1051
Kähler, K.C., Hassel, J.C., Heinzerling, L., Loquai, C., Mössner, R., Ugurel, S., . . . Gutzmer, R. (2016). Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. Journal of the German Society of Dermatology, 14, 662–681. doi:10.1111/ddg.13047
Kumar, V., Chaudhary, N., Garg, M., Floudas, C.S., Soni, P., & Chandra, A.B. (2017). Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Frontiers in Pharmacology, 8, 49. doi:10.3389/fphar.2017.00049
Lalla, R.V., Bowen, J., Barasch, A., Elting, L., Epstein, J., Keefe, D.M., . . . Elad, S. (2014). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer, 120, 1453–1461. doi:10.1002/cncr.28592
Merck. (2017, February 17). Use with live attenuated vaccines [Standard response letter to Kathleen Marie Madden, NP].
Naidoo, J., Page, D.B., Li, B.T., Connell, L.C., Schindler, K., Lacouture, M.E., . . . Wolchok, J.D. (2015). Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of Oncology, 26, 2375–2391. doi:10.1093/annonc/mdv383
National Cancer Institute. (2010). Common Terminology Criteria for Adverse Events [v.4.03]. Retrieved from https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference…
Rassy, E.E., Kourie, H.R., Rizkallah, J., El Karak, F., Hanna, C., Chelala, D.N., & Ghosn, M. (2016). Immune checkpoint inhibitors renal side effects and management. Immunotherapy, 8, 1417–1425. doi:10.2217/imt-2016-0099
Spain, L., Diem, S., & Larkin, J. (2016). Management of toxicities of immune checkpoint inhibitors. Cancer Treatment Reviews, 44, 51–60. doi:10.1016/j.ctrv.2016.02.001
U.S. Food and Drug Administration. (2012). Risk evaluation and mitigation strategy (REMS). Retrieved from https://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInfo…
Van Sebille, Y.Z., Stansborough, R., Wardill, H.R., Bateman, E., Gibson, R.J., & Keefe, D.M. (2015). Management of mucositis during chemotherapy: From pathophysiology to pragmatic therapeutics. Current Oncology Reports, 17, 50. doi:10.1007/s11912-015-0474-9
Weber, J.S., Postow, M., Lao, C.D., & Schadendorf, D. (2016). Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist, 21, 1230–1240. doi:10.1634/theoncologist.2016-0055




